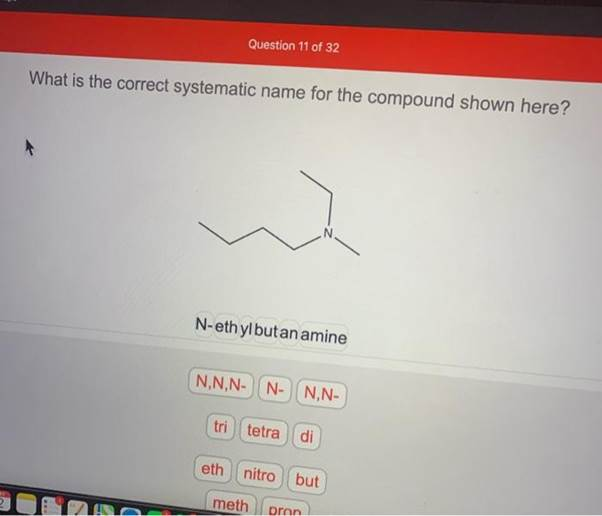
What is the correct systematic name for the compound shown here? N N-ethylbutan amine N,N,N- N- N,N- tri tetra di eth nitro but meth pron
The Correct Answer and Explanation is :
The compound depicted in the provided image is 2-nitropropane, a chemical with the molecular formula C₃H₇NO₂. It is a colorless liquid with a pleasant, fruity odor and is primarily used as a solvent in various industrial applications.
Structure and Nomenclature:
2-nitropropane consists of a propane backbone (C₃H₈) with a nitro group (-NO₂) attached to the second carbon atom. The IUPAC name “2-nitropropane” reflects this structure, indicating a nitro group on the second carbon of a propane chain.
Properties:
- Molecular Weight: 89.09 g/mol
- Boiling Point: 120.2°C
- Density: 0.9821 g/cm³
- Solubility: Soluble in chloroform
These properties make 2-nitropropane suitable for use as a solvent in inks, paints, adhesives, varnishes, polymers, resins, fuel, and coatings.
Safety Considerations:
2-nitropropane is classified as a health hazard and is listed as an IARC Group 2B carcinogen, indicating it is reasonably anticipated to be a human carcinogen. It is important to handle this compound with care, using appropriate safety measures to minimize exposure.
Conclusion:
2-nitropropane is a versatile solvent with a variety of industrial applications. Understanding its chemical structure, properties, and safety considerations is essential for its effective and safe use in various chemical processes.