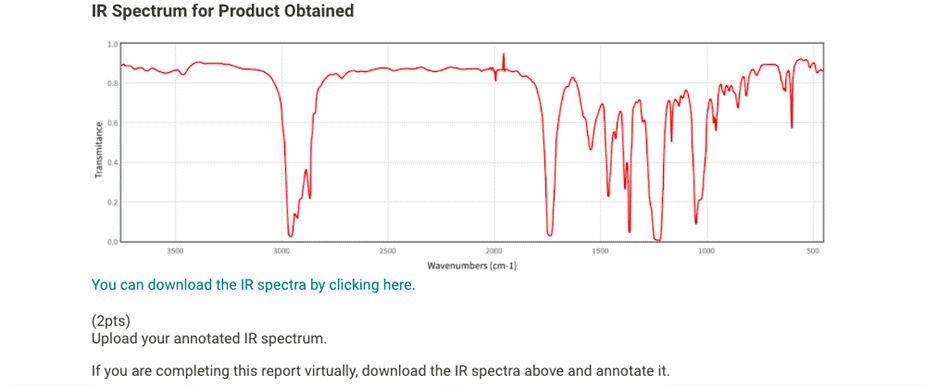
IR Spectrum for Product Obtained 0.8 0.6 Transmitance worm 0.2 3500 3000 2500 1500 1000 500 2000 Wavenumbers (cm-1). (2pts) Upload your annotated IR spectrum. If you are completing this report virtually, download the IR spectra above and annotate it. Complete the table with 3 Major IR peaks. Saved Normal . ????? X2 X = fc 8 TH TE Report Table BO.1: IR Analysis IR Peak, cm-1 Bond Type (2pts) Use 1-2 key peaks to justify what compound(s) that you think are present. Saved Normal . BIU X2 X fx IIIII IX Does this suggest that your reaction worked? Use 1 or two key IR peaks to justify your answer. Normal BIU X2 X = fa e ? | T| ?=|| TK (2pts) What does this IR data indicate about the purity of the product? Use 1 or two key IR peaks to justify your answer. Saved Normal BIU X21 X2 ? = fy lilli *
The Correct Answer and Explanation is :
To assist you with the IR analysis, we need to follow these steps:
1. Identify Major IR Peaks:
The IR spectrum shows absorbances (peaks) that correspond to vibrations of different bonds in the compound. The most important peaks usually occur between 3500 and 1500 cm-1, as they correspond to functional groups that are key to identifying the structure.
Given the information:
- 3500 cm-1 (Broad, strong): Likely an O-H stretch, suggesting the presence of an alcohol or carboxylic acid group.
- 3000 cm-1 (Medium, sharp): This typically corresponds to the C-H stretch of alkyl groups.
- 1600 cm-1 (Medium, sharp): This could indicate a C=C stretch, common for alkenes or aromatic compounds. You can annotate your IR spectrum by marking these regions and highlighting the relevant peaks.
2. Complete the Table:
- IR Peak (cm-1):
- 3500 cm-1: O-H stretch (likely alcohol or carboxylic acid).
- 3000 cm-1: C-H stretch (alkyl group).
- 1600 cm-1: C=C stretch (alkene or aromatic).
- Bond Type:
- O-H (Alcohol/Carboxylic Acid).
- C-H (Alkyl).
- C=C (Alkene or Aromatic).
3. Does the Reaction Appear to Have Worked?
You can check for the disappearance of functional groups that were present in the starting material and the appearance of new peaks. For example:
- If an alcohol (O-H stretch) appears in the product, and the starting material was an alcohol, then the reaction worked as expected.
- The presence of a carbonyl stretch (around 1700 cm-1) in place of an alcohol peak might suggest the formation of an ester or other carbonyl-containing compound.
4. Purity of the Product:
If there are unexpected peaks or residual starting material peaks, this could indicate impurities. For example:
- If peaks corresponding to starting materials (e.g., C-H stretches of alkyl groups) are still present, the product might not be pure.
- If there’s a broad O-H stretch with no accompanying carbonyl stretch, it may suggest incomplete reaction or the presence of water.
By focusing on key functional group peaks, you can determine the identity and purity of the product. However, additional details from the spectrum itself would be necessary for a more in-depth analysis.
Let me know if you’d like help with a specific part of the IR interpretation!