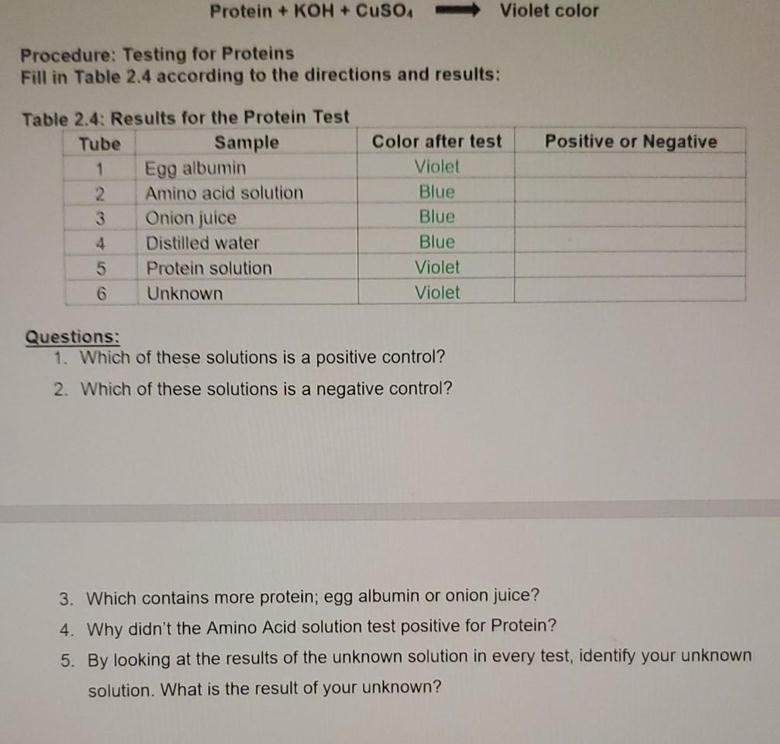
Testing for Proteins Fill in Table 2.4 according to the directions and results: Positive or Negative Table 2.4: Results for the Protein Test Tube Sample 1 Egg albumin 2. Amino acid solution 3 Onion juice 4 Distilled water Protein solution 6 Unknown Color after test Violet Blue Blue Blue Violet Violet Questions: Which of these solutions is a positive control? 2. Which of these solutions is a negative control? 3. Which contains more protein; egg albumin or onion juice? 4. Why didn't the Amino Acid solution test positive for Protein? 5. By looking at the results of the unknown solution in every test, identify your unknown solution. What is the result of your unknown? 1. The monomers that make up carbohydrates are: 2. What chemical is used to test for lipids? 3. The monomers that make up proteins are: 4. Which test would you use to distinguish between diet soda and regular soda? a. What would this test detect? 5. What chemical is used to test for starch? 6. Why was it important to include a water-only sample in each test? 7. Why was it important to wash out the test tubes in between tests? 8. The monomers that make up lipids are: 9. What does BMR stand for? 10. What is a Calorie? 11. What chemical is used to test for simple sugars? 12. What is the difference between saturated and unsaturated fats at room temperature? 13. What is metabolism?

The Correct Answer and Explanation is :
Table 2.4: Protein Test Results
| Test Tube | Sample | Color After Test |
|---|---|---|
| 1 | Egg albumin | Violet |
| 2 | Amino acid solution | Blue |
| 3 | Onion juice | Blue |
| 4 | Distilled water | Blue |
| 5 | Protein solution | Violet |
| 6 | Unknown | Violet |
Answers to Questions:
- Which of these solutions is a positive control?
Egg albumin (Sample 1) and protein solution (Sample 5) are positive controls, as they both show a violet color after the test, indicating the presence of proteins. - Which of these solutions is a negative control?
Distilled water (Sample 4) is the negative control, as it shows a blue color, indicating no proteins present. - Which contains more protein: egg albumin or onion juice?
Egg albumin contains more protein. This is supported by its violet color result in the protein test, which is indicative of a high concentration of proteins compared to the blue color of onion juice. - Why didn’t the amino acid solution test positive for protein?
The amino acid solution didn’t test positive because amino acids are the building blocks of proteins, but not proteins themselves. The test for proteins detects the peptide bonds between amino acids, not individual amino acids. Therefore, the test doesn’t detect a positive result with the amino acid solution. - By looking at the results of the unknown solution in every test, identify your unknown solution. What is the result of your unknown?
Based on the result (violet), the unknown solution likely contains a protein. It matches the positive result for protein found in egg albumin and the protein solution.
Additional Questions:
- The monomers that make up carbohydrates are:
Monosaccharides, such as glucose and fructose. - What chemical is used to test for lipids?
The Sudan III test is used to detect lipids, as it stains lipids red or orange. - The monomers that make up proteins are:
Amino acids. - Which test would you use to distinguish between diet soda and regular soda? What would this test detect?
The Benedict’s test for reducing sugars would be used. Regular soda contains sugars like sucrose, which will react positively (changing color), while diet soda will not. - What chemical is used to test for starch?
Iodine solution is used to test for starch. If starch is present, the iodine will turn blue-black. - Why was it important to include a water-only sample in each test?
The water-only sample serves as a negative control to ensure that the reagents are functioning properly and that any observed color changes are due to the test substances and not contamination. - Why was it important to wash out the test tubes in between tests?
Washing out the test tubes between tests prevents contamination from previous substances, ensuring accurate and reliable results. - The monomers that make up lipids are:
Fatty acids and glycerol. - What does BMR stand for?
Basal Metabolic Rate. - What is a Calorie?
A Calorie (with a capital “C”) is a unit of energy. It is the amount of heat required to raise the temperature of one kilogram of water by one degree Celsius. - What chemical is used to test for simple sugars?
Benedict’s solution is used to test for simple sugars (reducing sugars). - What is the difference between saturated and unsaturated fats at room temperature?
Saturated fats are solid at room temperature, while unsaturated fats are liquid. Saturated fats have no double bonds between carbon atoms, while unsaturated fats have one or more double bonds. - What is metabolism?
Metabolism is the set of life-sustaining chemical reactions in organisms that converts food into energy, supports growth, and maintains bodily functions.
300-Word Explanation:
In this experiment, different biological macromolecules are tested using specific reagents that react with proteins, lipids, carbohydrates, and other substances. A positive control is a known substance that will give a positive result, and a negative control gives no reaction. For protein detection, the reagent used (likely biuret reagent) turns violet in the presence of protein, confirming its presence. Egg albumin and the protein solution test positive, while onion juice and distilled water do not, showing the importance of understanding positive and negative controls in experimental design. The amino acid solution doesn’t test positive because individual amino acids don’t form the complex structures that the reagent detects.
Monomers such as amino acids and monosaccharides combine to form proteins and carbohydrates, respectively. The chemical tests for starch and lipids detect the presence of these substances in the samples, with iodine turning starch blue-black and Sudan III staining lipids red. The experiment also emphasizes the importance of washing test tubes to avoid cross-contamination between tests and the role of water-only samples in ensuring accurate baseline measurements.
Metabolism, which involves the breakdown of food into usable energy, underpins all of these tests, as the nutrients detected serve as vital components for energy production and growth. Understanding how these tests detect biological macromolecules helps in various fields such as health, nutrition, and biochemistry.