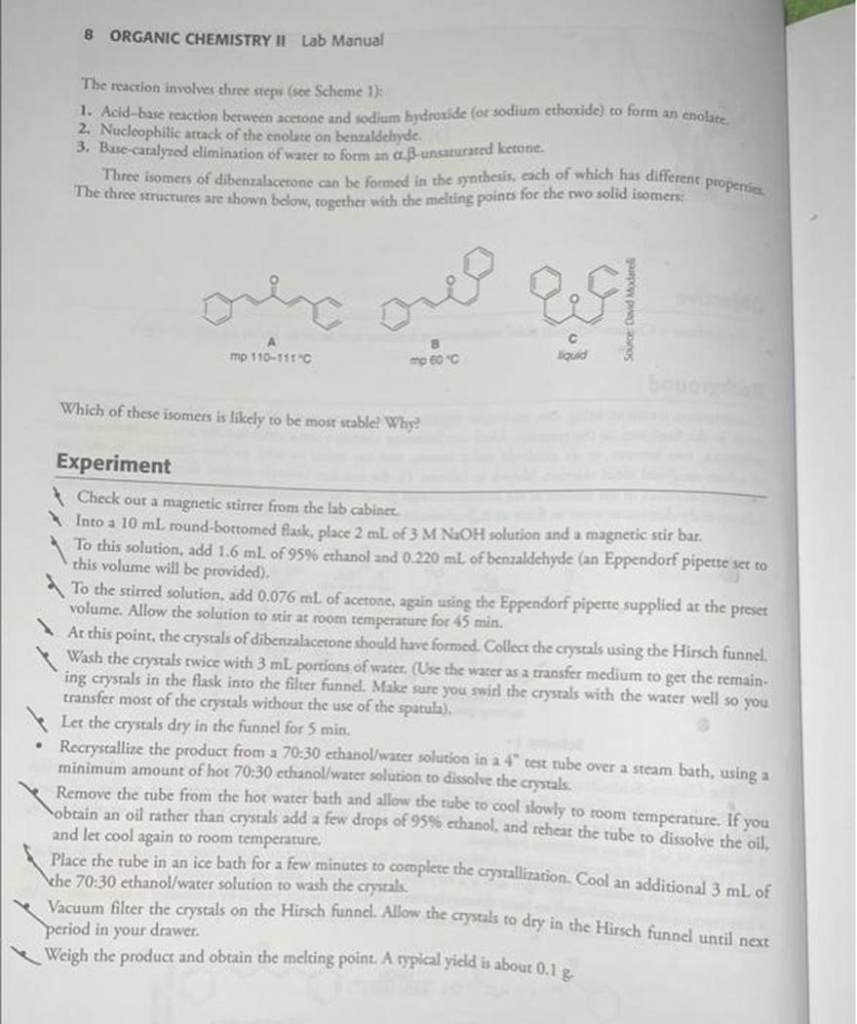
The weight of the dibenzalacetone crystals was 0.150 grams. The melting point for the dibenzalacetone crystals was 108-110 degrees Celcius. Determine the adjusted stoichiometry and the limitimg reagent. Then determine the theoretical yeild and the percent yeild.
The Correct Answer and Explanation is :
Dibenzalacetone is synthesized through an aldol condensation reaction between benzaldehyde and acetone. In this reaction, two moles of benzaldehyde react with one mole of acetone to form dibenzalacetone. The balanced chemical equation is:
[ 2 \, \text{C}_6\text{H}_5\text{CHO} + \text{CH}_3\text{COCH}_3 \rightarrow \text{C}_6\text{H}_5\text{CH}=\text{CH}\text{COCH}=\text{CH}\text{C}_6\text{H}_5 ]
Determining the Limiting Reagent:
To identify the limiting reagent, we need to calculate the moles of each reactant used:
- Benzaldehyde (C₆H₅CHO):
- Molar mass = 106.13 g/mol
- Mass used = 0.82 g
- Moles = 0.82 g ÷ 106.13 g/mol ≈ 0.00772 mol
- Acetone (CH₃COCH₃):
- Molar mass = 58.08 g/mol
- Mass used = 0.24 g
- Moles = 0.24 g ÷ 58.08 g/mol ≈ 0.00413 mol
According to the stoichiometry of the reaction, two moles of benzaldehyde react with one mole of acetone. Therefore, for 0.00413 mol of acetone, 0.00826 mol of benzaldehyde is required. Since only 0.00772 mol of benzaldehyde is available, benzaldehyde is the limiting reagent.
Calculating the Theoretical Yield:
The theoretical yield is determined by the amount of product that can be formed from the limiting reagent. Since the molar ratio of benzaldehyde to dibenzalacetone is 2:1, 0.00772 mol of benzaldehyde will produce 0.00386 mol of dibenzalacetone.
- Molar mass of dibenzalacetone = 234 g/mol
- Theoretical yield = 0.00386 mol × 234 g/mol ≈ 0.905 g
Calculating the Percent Yield:
The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100:
- Actual yield = 0.150 g
- Percent yield = (0.150 g ÷ 0.905 g) × 100 ≈ 16.6%
Conclusion:
In this experiment, benzaldehyde is the limiting reagent, and the theoretical yield of dibenzalacetone is approximately 0.905 g. The actual yield obtained is 0.150 g, resulting in a percent yield of about 16.6%. This relatively low yield may be attributed to factors such as incomplete reactions, losses during product isolation, or impurities in the reagents.