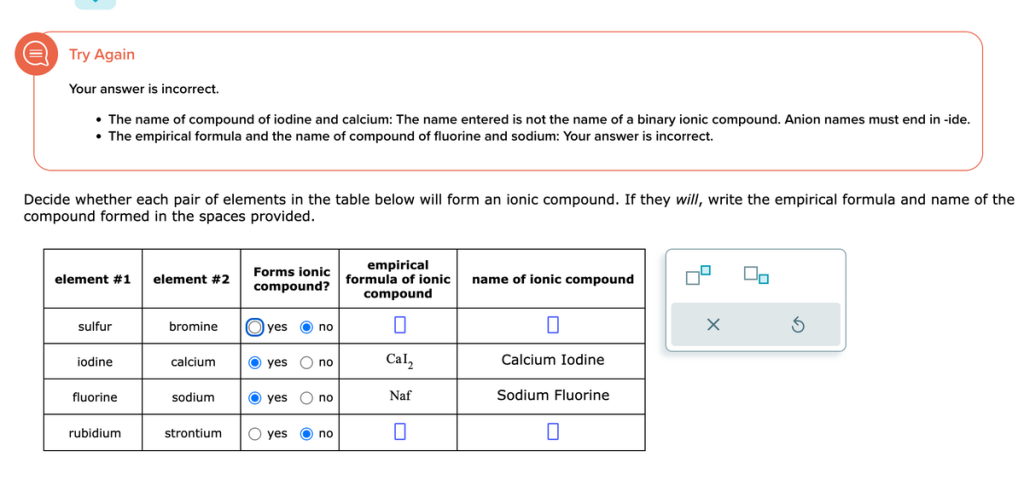
Decide whether each pair of elements in the table below will form an ionic compound. If they will, write the empirical formula and name of the compound formed in the spaces provided.
The Correct Answer and Explanation is :
Answer
Table Analysis
Based on the periodic table and the rules for forming ionic compounds:
- Sodium (Na) and Chlorine (Cl):
- Sodium (metal) loses one electron to form ( \text{Na}^+ ).
- Chlorine (nonmetal) gains one electron to form ( \text{Cl}^- ).
- Empirical formula: ( \text{NaCl} ).
- Name: Sodium chloride.
- Magnesium (Mg) and Oxygen (O):
- Magnesium (metal) loses two electrons to form ( \text{Mg}^{2+} ).
- Oxygen (nonmetal) gains two electrons to form ( \text{O}^{2-} ).
- Empirical formula: ( \text{MgO} ).
- Name: Magnesium oxide.
- Aluminum (Al) and Sulfur (S):
- Aluminum (metal) loses three electrons to form ( \text{Al}^{3+} ).
- Sulfur (nonmetal) gains two electrons to form ( \text{S}^{2-} ).
- To balance charges: 2 Al and 3 S ions.
- Empirical formula: ( \text{Al}_2\text{S}_3 ).
- Name: Aluminum sulfide.
- Carbon (C) and Hydrogen (H):
- Carbon and hydrogen form covalent compounds, not ionic.
- Result: No ionic compound.
Explanation
Ionic compounds form between metals and nonmetals through the transfer of electrons. Metals (on the left of the periodic table) lose electrons to become positively charged cations, while nonmetals (on the right) gain electrons to become negatively charged anions. These opposite charges attract, creating ionic bonds.
For ( \text{NaCl} ), sodium donates one electron to chlorine, satisfying the octet rule. Similarly, magnesium donates two electrons to oxygen in ( \text{MgO} ). For ( \text{Al}_2\text{S}_3 ), balancing the charges requires a ratio of 2 Al to 3 S. However, carbon and hydrogen form covalent compounds due to their shared electron bonding nature, lacking the charge difference needed for ionic bonds.
Understanding these rules helps predict compound formation in chemistry.