Draw all resonance structures for the nitric seld molecule, HNO, (NO₂OH) Explicitly drew all atoms. Inchade all valence lone pairs sis your answer Do not include overall ion charges or formal charges in your drawing. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner Separate resonance structures using the symbol from the drop-down menu
The Correct Answer and Explanation is :
To help you visualize the resonance structures for the nitric acid molecule (HNO₃, also written as HNO₂OH), I’ll first explain the structure and how resonance works in this case, then describe how to draw each resonance form. In this case, we’ll avoid adding formal charges and stick to just drawing the valence electrons.
General Structure of Nitric Acid (HNO₃):
- Central Atom: Nitrogen (N) is the central atom.
- Surrounding Atoms: Three oxygen atoms are bonded to nitrogen, one of which is bonded to a hydrogen atom (the hydroxyl group). The other two oxygen atoms are part of a nitro group (-NO₂).
- Valence Electrons: Nitrogen has 5 valence electrons, oxygen has 6, and hydrogen has 1.
Resonance Structures:
Resonance arises when there are multiple ways to arrange electrons within a molecule without changing the overall connectivity of the atoms. For nitric acid, the structure involves alternating bonds between nitrogen and oxygen.
1st Resonance Structure:
- Nitrogen is bonded to three oxygens: one through a single bond with a hydroxyl group (OH), and the other two are double-bonded with the nitrogen.
- One oxygen has a single bond to nitrogen and a single bond to hydrogen (OH).
- The other two oxygens form double bonds with nitrogen, which respects the octet rule.
2nd Resonance Structure:
- In this structure, the double bond between nitrogen and one oxygen is moved to a single bond, and the single bond between nitrogen and another oxygen becomes a double bond.
- This maintains the overall connectivity, but the distribution of electrons has shifted.
3rd Resonance Structure:
- A similar shift happens, but this time the oxygen connected to the hydrogen (OH) switches its bond with the nitrogen, creating a structure with different electron distribution while maintaining the integrity of the bonds.
These resonance structures are necessary because nitrogen does not have enough electrons to satisfy its octet if all bonds were single or all were double. The resonance structures help distribute the electrons in a way that allows for more stable bonding.
Now, I will draw the resonance structures.
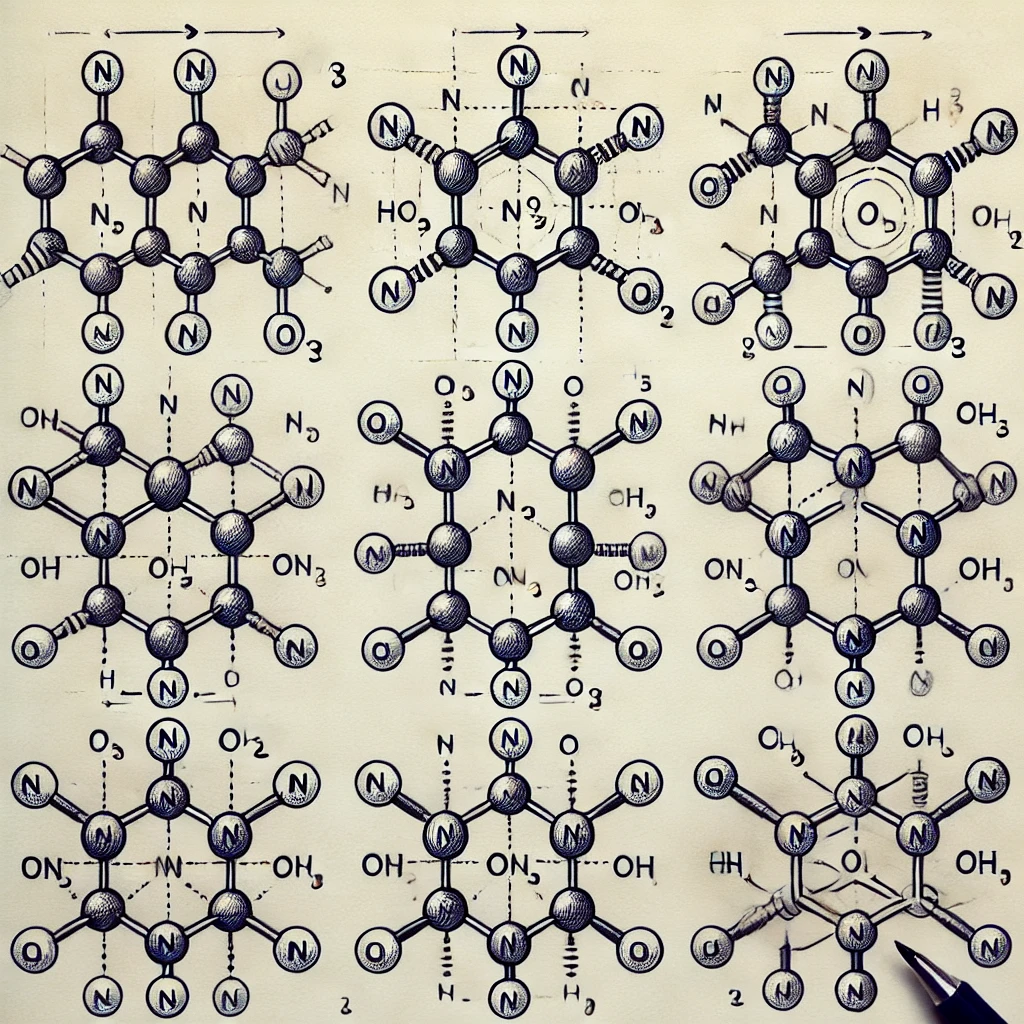
Here are the three resonance structures of the nitric acid molecule (HNO₃), which show how the bonding between nitrogen (N) and oxygen (O) changes. Each structure represents a different distribution of electrons that satisfies the octet rule for nitrogen and oxygen atoms. Notice the alternating single and double bonds between nitrogen and oxygen, along with lone pairs on oxygen atoms, which reflect the resonance behavior.
In summary:
- First Structure: The nitrogen is double-bonded to two oxygens and single-bonded to a hydroxyl group (OH).
- Second Structure: The nitrogen-oxygen bond shifts so that one nitrogen-oxygen double bond becomes a single bond, and another nitrogen-oxygen single bond becomes a double bond.
- Third Structure: A similar shift in bonds occurs, continuing the resonance.
These structures highlight how the electron distribution in the molecule can be described with multiple valid representations.