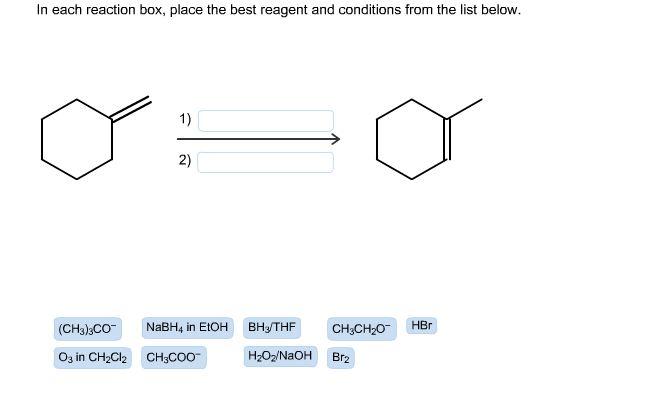
In each reaction box, place the best reagent and conditions from the list below. CH3CH20- HBr (CH3) 3CO NaBH4 in EtoH BH3 THF H202 NaOH Br2 O3 in CH2C12 CH3COO-
The Correct Answer and Explanation is :
It appears you’re working on a chemical reaction question, but the image link you provided does not seem to work. Without the specific reaction scheme, I can help guide you on how to approach the problem with general advice on each reagent.
Here’s an overview of the reagents listed, along with their typical uses and the conditions they might be applied under:
- CH3CH2O- (Ethoxide): This is a strong nucleophile and base commonly used for nucleophilic substitution reactions (SN2) or elimination reactions (E2). It is typically used in alcoholic solvents and might be used for dehydrohalogenation or to form alkoxide ions from alcohols.
- HBr (Hydrobromic acid): HBr is a strong acid and is typically used for electrophilic addition reactions, such as the addition of HBr to alkenes (Markovnikov addition), or for substitution reactions with alkyl halides in SN1 or SN2 mechanisms.
- (CH3)3CO (tert-butoxide): A bulky base that is often used in elimination reactions (E2) or to create hindered products due to its steric bulk. It can deprotonate alcohols or alkyl halides in the presence of strong conditions.
- NaBH4 in EtOH (Sodium borohydride in ethanol): A mild reducing agent that typically reduces carbonyl compounds like aldehydes and ketones to alcohols. It is selective, and in ethanol, it will often work to reduce ketones or aldehydes without reducing esters or carboxylic acids.
- BH3 (Borane): Used as a reducing agent, particularly for hydroboration of alkenes. This reagent adds across double bonds in a syn fashion to form organoborane intermediates, which can later be oxidized to alcohols using H2O2.
- THF (Tetrahydrofuran): A solvent commonly used for reactions involving organometallic reagents (like Grignard or lithium reagents), or with BH3, NaBH4, or LiAlH4. It is an aprotic solvent that helps dissolve non-polar reagents.
- H2O2 (Hydrogen peroxide): A strong oxidizing agent. In the context of reactions with borane (BH3), H2O2 is often used to oxidize the borane intermediates to alcohols.
- NaOH (Sodium hydroxide): A strong base used for deprotonation, neutralization, and certain elimination (E2) or condensation reactions. It is also used in the saponification of esters or hydrolysis reactions.
- Br2 (Bromine): A halogen used for addition reactions to alkenes, leading to vicinal dibromides, or for electrophilic halogenation reactions in aromatic compounds.
- O3 in CH2Cl2 (Ozone in dichloromethane): Used for ozonolysis of alkenes, where the double bond is cleaved and two carbonyl compounds are formed.
- CH3COO- (Acetate ion): A moderate nucleophile, often used in nucleophilic substitution reactions (SN2), where it displaces a leaving group, such as in ester formation from acyl chlorides.
Given these reagents, the correct combination for each reaction will depend on the transformation you’re aiming to achieve (such as nucleophilic substitution, reduction, oxidation, etc.).
If you can describe the specific reactions or transformations in the image, I can provide more detailed instructions on the optimal reagents and conditions for each one.