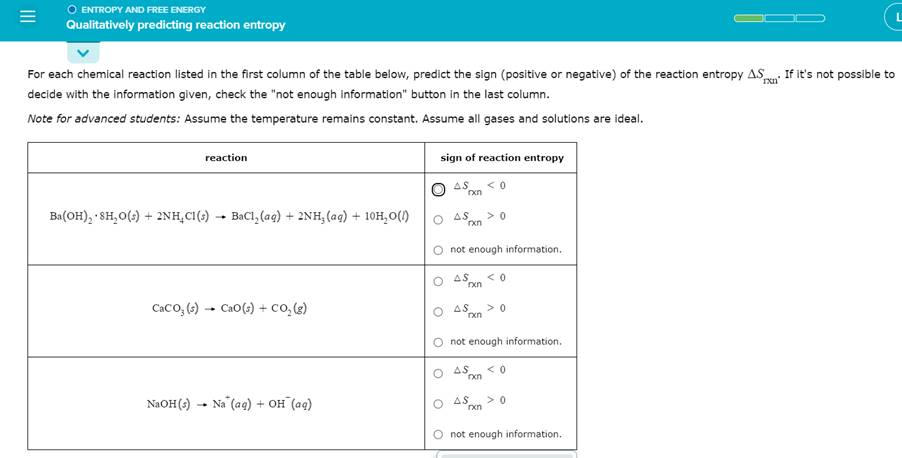
O ENTROPY AND FREE ENERGY Qualitatively predicting reaction entropy L For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy ASixn. If it’s not possible to decide with the information given, check the “not enough information” button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction sign of reaction entropy AS < 0=”” rxn=”” ba(oh)2=”” ·=”” 8h,0(5)=”” +=”” 2nh,=”” c1(s)=”” bacl,=”” (aq)=”” +=”” 2nh2=”” (aq)=”” +=”” 10h,0(1)=”” as=””> 0 rxn O not enough information. ? ?S. < 0=”” rxn=”” cacoz=”” (s)=”” -=”” cao(s)=”” +=”” co2(g)=”” as=””> 0 rxn O not enough information. AS < 0=”” rxn=”” naoh(s)=”” -=”” na=”” (aq)=”” +=”” oh=”” (aq)=”” o=”” as=””> 0 G?n O not enough information.
The Correct Answer and Explanation is :
To predict the sign of the reaction entropy ((\Delta S_{\text{rxn}})) for each chemical reaction, we analyze the changes in the system’s disorder or randomness. Entropy increases ((\Delta S > 0)) when the number of gas molecules or solute particles increases or when solid or liquid phases transition to gases. Conversely, entropy decreases ((\Delta S < 0)) when the system becomes more ordered, such as when gases condense or solutes precipitate.
1. ( \text{Ba(OH)}_2 \cdot 8\text{H}_2\text{O (s)} + 2\text{NH}_4\text{Cl (s)} \rightarrow \text{BaCl}_2(\text{aq}) + 2\text{NH}_3(\text{aq}) + 10\text{H}_2\text{O}(\ell) )
Prediction: (\Delta S > 0)
This reaction involves the dissolution of solids ((\text{Ba(OH)}_2 \cdot 8\text{H}_2\text{O}) and (\text{NH}_4\text{Cl})) into aqueous and liquid components ((\text{BaCl}_2), (\text{NH}_3), and (\text{H}_2\text{O})). The increase in the number of aqueous particles and the breakdown of solid structures significantly increases the system’s randomness.
2. ( \text{CaCO}_3 (\text{s}) \rightarrow \text{CaO}(\text{s}) + \text{CO}_2(\text{g}) )
Prediction: (\Delta S > 0)
The decomposition of a solid ((\text{CaCO}_3)) produces a solid ((\text{CaO})) and a gas ((\text{CO}_2)). The formation of the gas significantly increases the entropy because gases have much higher randomness compared to solids.
3. ( \text{NaOH}(\text{s}) \rightarrow \text{Na}^+(\text{aq}) + \text{OH}^-(\text{aq}) )
Prediction: (\Delta S > 0)
This reaction describes the dissolution of solid (\text{NaOH}) into its ions in aqueous solution. The process increases the disorder of the system because the rigid structure of the solid is broken into mobile ions in solution.
Explanation
Entropy changes ((\Delta S)) are governed by changes in the system’s degree of randomness or disorder. Processes that involve the formation of gases, the dissolution of solids, or the increase in the number of particles generally lead to positive entropy changes ((\Delta S > 0)). Conversely, processes that reduce particle motion or condense gases tend to decrease entropy ((\Delta S < 0)). In all three reactions, the system’s randomness increases, resulting in (\Delta S > 0) for each case.