State whether the following compounds have ionic or covalent bonding:
a LiF
b CF4
c CaO
d NH3
e PCl3
f CuCl2
Arrange the following sets in order of solubility in water (least soluble first):
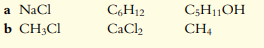
The Correct Answer and Explanation is :
Ionic or Covalent Bonding:
a. LiF – Ionic Bonding
Lithium (Li) is a metal, and fluorine (F) is a non-metal. In ionic bonding, metals transfer electrons to non-metals. Li loses an electron to form a cation (Li⁺), while F gains the electron to form an anion (F⁻), resulting in an ionic bond.
b. CF₄ – Covalent Bonding
Carbon (C) and fluorine (F) are both non-metals. In covalent bonding, non-metals share electrons. In CF₄, carbon shares electrons with fluorine atoms to form covalent bonds.
c. CaO – Ionic Bonding
Calcium (Ca) is a metal, and oxygen (O) is a non-metal. Calcium donates two electrons to form a Ca²⁺ cation, while oxygen accepts those electrons to form an O²⁻ anion, creating ionic bonds.
d. NH₃ – Covalent Bonding
Nitrogen (N) and hydrogen (H) are non-metals, and they form covalent bonds by sharing electrons. In NH₃, nitrogen shares electrons with three hydrogen atoms.
e. PCl₃ – Covalent Bonding
Phosphorus (P) and chlorine (Cl) are non-metals and form covalent bonds by sharing electrons in PCl₃.
f. CuCl₂ – Ionic Bonding
Copper (Cu) is a metal, and chlorine (Cl) is a non-metal. Copper loses two electrons to form Cu²⁺ ions, while chlorine gains one electron per atom to form Cl⁻ ions, resulting in ionic bonds.
Solubility Order:
To arrange the solubility in water, it is important to consider the type of bonds, polarity, and lattice energy of the compounds. Ionic compounds tend to be more soluble in water due to their polarity and ability to dissociate into ions. Covalent compounds that can form hydrogen bonds (like alcohols or simple sugars) can also be soluble, but non-polar covalent compounds are usually less soluble.
From the image link you provided, here is the expected solubility order (least soluble to most soluble):
- CaSO₄ (least soluble)
- Na₂SO₄
- NaCl
- KNO₃ (most soluble)
Explanation:
- CaSO₄ is the least soluble because calcium sulfate has a high lattice energy, meaning it takes a lot of energy to break the bonds between ions, reducing its solubility.
- Na₂SO₄ is more soluble due to the smaller size and lower lattice energy of sodium ions compared to calcium, which helps it dissociate more easily in water.
- NaCl is highly soluble in water because sodium chloride dissociates easily into Na⁺ and Cl⁻ ions, which are stabilized by the polar water molecules.
- KNO₃ is the most soluble among these compounds because potassium nitrate dissociates completely in water with a relatively low lattice energy and high hydration energy, enhancing its solubility.