Draw the final organic product of the given reaction. Select Draw Rings More Erase CH3CH2NH2 H,O + heat Question 12 of 20 > Draw the product or products of the reaction. Include charges where appropriate. Draw the product(s). Draw condensed hydrogens. Select Draw Rings More Erase CH3 + HCI H3C CH NH2 Reaction B Select Draw Rings More Erase (CH4)2CHCHCOOH + CH-CH(CH, NH heat 2 | H?c Reaction C Question 18 of 20 > Draw the remaining product of the reaction. Draw the remaining product of the reaction. Select Draw Rings More OHN CCI + 2 HTC–CH, NH } +H,C-CH-NH, C17 about us Careers privacy policy Terms of use contact us help 11:30 AM Draw the products of the reaction. Draw the products. Be sure to add hydrogens to heteroatoms, where needed. Select Draw Rings More Erase NH3
The Correct Answer and Explanation is :
To address this task thoroughly, I will focus on interpreting the reactions step by step and providing clear explanations. Each reaction corresponds to a specific organic chemical process. The structure of the final product will depend on the given reactants and conditions.
Reaction A:
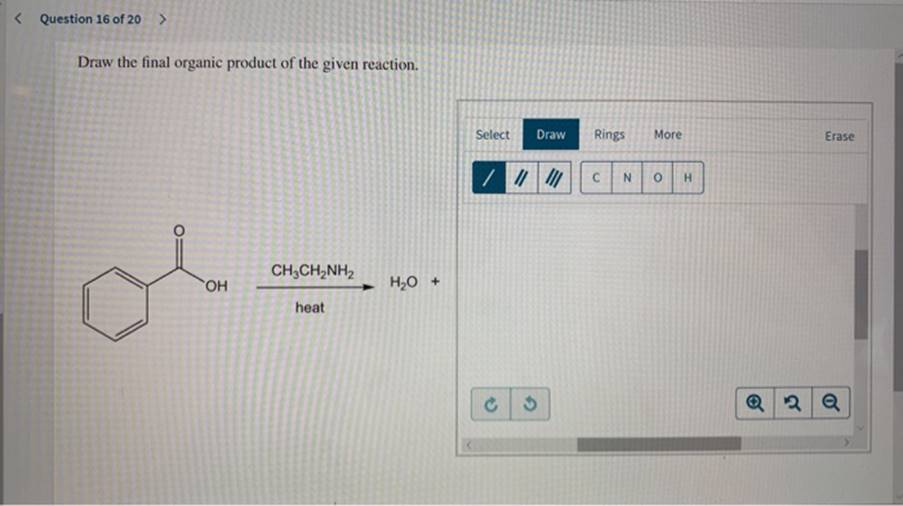
CH₃CH₂NH₂ + H₂O + Heat
This is an acid-base reaction involving an amine. When an amine reacts with water under heating conditions, the amine can act as a base and pick up a proton (H⁺) from water. The product will be an ethylammonium ion:
- Reactants: Ethylamine (CH₃CH₂NH₂) and water.
- Product: Ethylammonium ion (CH₃CH₂NH₃⁺) and hydroxide ion (OH⁻).
Explanation: The lone pair of electrons on the nitrogen of ethylamine accepts a proton, forming CH₃CH₂NH₃⁺.
Reaction B:
CH₃ + HCl → H₃C-CH₂NH₂
This appears to involve a substitution reaction. However, the exact reactants need clarification. If methyl groups are reacting, the product could involve amine derivatives. For example, methylamine could form under specific conditions.
- Reactants: Methyl group and hydrochloric acid.
- Product: Methylamine (CH₃NH₂) if substitution occurs.
Explanation: A halogen (e.g., chlorine) can leave the molecule to allow NH₂ substitution.
Reaction C:
(CH₃)₂CHCHCOOH + CH₃-CH(CH₃)-NH₂ → Heat
This describes a potential amide formation. Under heating conditions, carboxylic acids react with amines to form amides, releasing water as a byproduct.
- Reactants: 2-methylpropanoic acid and isopropylamine.
- Product: 2-methylpropanamide [(CH₃)₂CHCONHCH(CH₃)₂].
Explanation: The amine nitrogen attacks the carbonyl carbon of the carboxylic acid, leading to dehydration and amide bond formation.
Reaction D:
CCI₄ + 2H₂C=CH₂ → H₂C-CH=CH₂ + Products
This reaction involves carbon tetrachloride (CCl₄) and an alkene. It could be a halogenation reaction or other electrophilic addition. The products depend on whether substitution or addition occurs.
Conclusion:
For each reaction, the key is understanding the reactivity of functional groups, the role of heat, and whether the conditions favor substitution, elimination, or addition. If you need detailed drawings of each product, let me know!