Construct the molecular orbital diagram for H2- and then identify the bond order.
The Correct Answer and Explanation is :
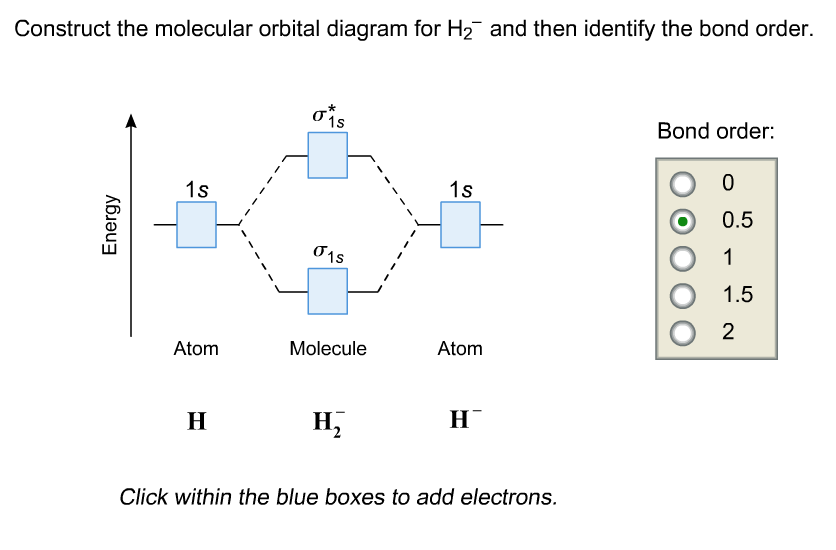
Molecular Orbital Diagram for ( \text{H}_2^- )
To construct the molecular orbital (MO) diagram for ( \text{H}_2^- ) (a hydrogen molecule with one extra electron), follow these steps:
- Atomic Orbitals: Each hydrogen atom contributes one 1s atomic orbital. These combine to form molecular orbitals.
- Formation of Molecular Orbitals:
- The constructive interference of 1s orbitals forms a bonding molecular orbital (( \sigma_{1s} )).
- The destructive interference forms an antibonding molecular orbital (( \sigma_{1s}^* )).
- Electron Configuration:
- A neutral ( \text{H}2 ) molecule has 2 electrons, which fill the lower-energy ( \sigma{1s} ) orbital.
- In ( \text{H}2^- ), the extra electron is added to the higher-energy ( \sigma{1s}^* ) orbital.
Molecular Orbital Diagram:
- Energy Levels:
- ( \sigma_{1s} ) (lower energy, bonding)
- ( \sigma_{1s}^* ) (higher energy, antibonding)
- Electron Distribution:
- ( \sigma_{1s} ): 2 electrons
- ( \sigma_{1s}^* ): 1 electron
Bond Order Calculation:
The bond order is given by the formula:
[
\text{Bond Order} = \frac{\text{Number of bonding electrons} – \text{Number of antibonding electrons}}{2}
]
- Bonding electrons = 2 (in ( \sigma_{1s} ))
- Antibonding electrons = 1 (in ( \sigma_{1s}^* ))
[
\text{Bond Order} = \frac{2 – 1}{2} = 0.5
]
Explanation:
The ( \text{H}_2^- ) ion has a bond order of 0.5, indicating a weaker bond than neutral ( \text{H}_2 ) (bond order = 1). The additional electron in the antibonding orbital partially cancels the stabilization provided by the bonding orbital. This bond is weaker and longer than in ( \text{H}_2 ), but the molecule still exists due to the net bonding interaction. This explains why ( \text{H}_2^- ) is less stable than ( \text{H}_2 ).