Complete the molecular orbital diagram for NF, nitrogen monofluoride below. Electrons can be added to the orbitals by clicking the orbital. Additional clicks will change the occupation of the orbital. Be sure to fill in both the atomic and molecular orbitals. Note: atomic orbitals are not shown.
The Correct Answer and Explanation is :
To solve the molecular orbital (MO) diagram for nitrogen monofluoride (NF), we analyze the electronic configuration and molecular orbital interactions between nitrogen (N) and fluorine (F). Here’s the correct answer and explanation:
Key Steps in Constructing the MO Diagram:
- Atomic Orbitals:
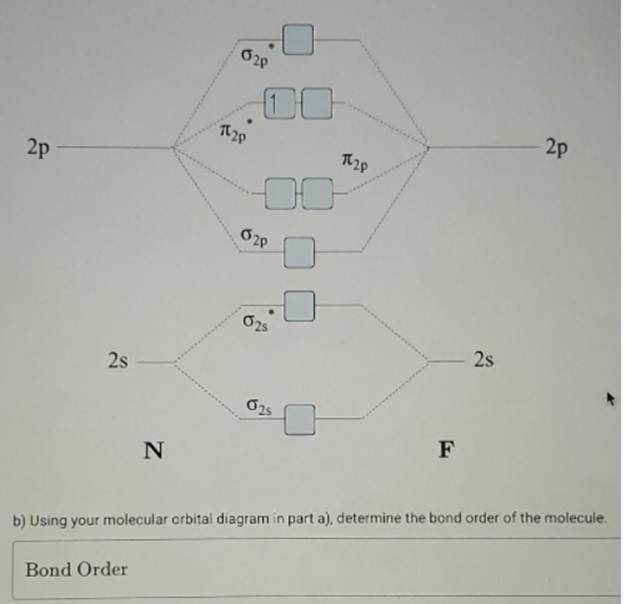
- Nitrogen has an electron configuration of ( 1s^2 2s^2 2p^3 ).
- Fluorine has an electron configuration of ( 1s^2 2s^2 2p^5 ).
- The 2s and 2p orbitals of N and F contribute to the molecular orbitals.
- Relative Energies of Atomic Orbitals:
- Fluorine is more electronegative than nitrogen, so its atomic orbitals are lower in energy.
- This energy difference affects the mixing of orbitals, with fluorine’s orbitals having greater influence in bonding orbitals.
- Molecular Orbitals:
- The molecular orbitals are formed by the linear combination of atomic orbitals (LCAO).
- The ordering of MOs for diatomic molecules involving second-period elements like N and F follows the standard order:
- ( \sigma(2s) ), ( \sigma^(2s) ), ( \pi(2p_x, 2p_y) ), ( \sigma(2p_z) ), ( \pi^(2p_x, 2p_y) ), ( \sigma^*(2p_z) ).
- Filling the Molecular Orbitals:
- Total valence electrons in NF = 5 (N) + 7 (F) = 12.
- These 12 electrons fill the molecular orbitals in increasing energy:
- ( \sigma(2s) ): 2 electrons
- ( \sigma^*(2s) ): 2 electrons
- ( \pi(2p_x) ) and ( \pi(2p_y) ): 4 electrons (2 in each)
- ( \sigma(2p_z) ): 2 electrons
- ( \pi^(2p_x) ) and ( \pi^(2p_y) ): Remaining 2 electrons
Explanation of Stability and Bonding:
- The bond order can be calculated as:
[
\text{Bond Order} = \frac{(\text{Number of bonding electrons} – \text{Number of antibonding electrons})}{2}
]
In NF:
[
\text{Bond Order} = \frac{8 – 4}{2} = 2
] - A bond order of 2 indicates a double bond, implying significant bonding strength between N and F.
This diagram illustrates the partial ionic character (due to fluorine’s higher electronegativity) and explains the molecule’s bonding and properties.