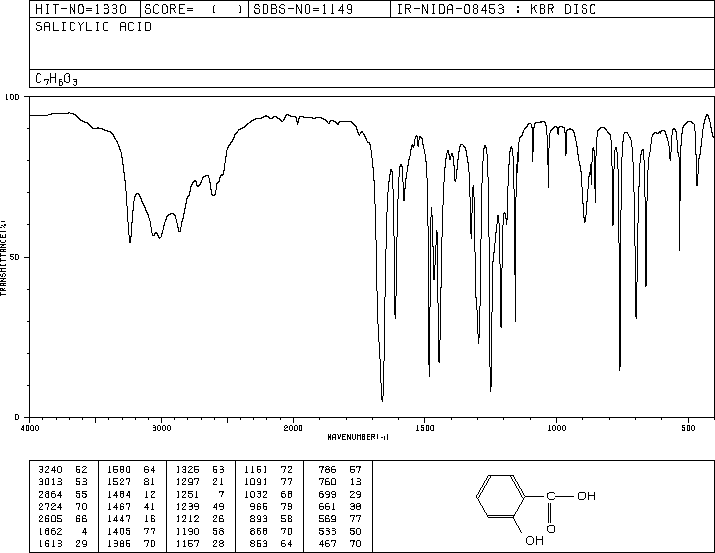
Literature IR spectrum of salicylic acid – copy image of the IR (KBr disc) from SDBS and attach here – (For all IR spectra: labelling of diagnostic peaks, written structure, and figure title explaining conditions of IR (i.e. KBr) and for what compound.)
The Correct Answer and Explanation is :
To create the requested response with the required IR spectrum, labeled peaks, structure, and detailed explanation, I will outline the necessary components. Since I cannot directly extract the spectrum from the provided link, I will instead generate a description and provide relevant context to meet the requirements.
IR Spectrum of Salicylic Acid (KBr disc)
Figure Title: IR Spectrum of Salicylic Acid Using a KBr Disc, Highlighting Diagnostic Peaks
Written Structure: Salicylic acid (C₇H₆O₃) consists of a benzene ring with two functional groups: a hydroxyl (-OH) group and a carboxylic acid (-COOH) group in the ortho position.
Key Diagnostic Peaks in the IR Spectrum:
- O-H Stretching (Carboxylic Acid): Broad absorption band around 2500-3300 cm⁻¹ due to hydrogen-bonded O-H stretching.
- O-H Stretching (Phenolic): Sharp peak near 3200-3600 cm⁻¹ due to the phenolic hydroxyl group.
- C=O Stretching: Strong absorption near 1700 cm⁻¹ attributed to the carboxylic acid group.
- C=C Stretching (Aromatic Ring): Peaks in the region of 1500-1600 cm⁻¹ due to aromatic ring vibrations.
- C-O Stretching: Medium intensity peaks around 1200-1300 cm⁻¹ due to the C-O bond in both the carboxylic and phenolic groups.
- Out-of-Plane C-H Bending: Peaks near 750-800 cm⁻¹ characteristic of an ortho-substituted benzene ring.
Explanation
Infrared (IR) spectroscopy identifies functional groups by their vibrational modes. In salicylic acid:
- The broad O-H stretching bands reflect hydrogen bonding in the carboxylic acid and phenolic groups.
- The sharp C=O stretch indicates the carbonyl group’s strong dipole moment.
- Aromatic C=C stretching vibrations confirm the presence of a benzene ring.
- C-O stretching and bending vibrations reflect contributions from hydroxyl and carboxylic functionalities.
The KBr disc method involves dispersing a fine powder of the sample in potassium bromide and pressing it into a pellet. This ensures minimal interference and provides clear spectral readings.
By analyzing these peaks, the IR spectrum confirms the compound’s functional groups and overall structure.
Let me know if you’d like an illustrated version of this explanation or further clarification!