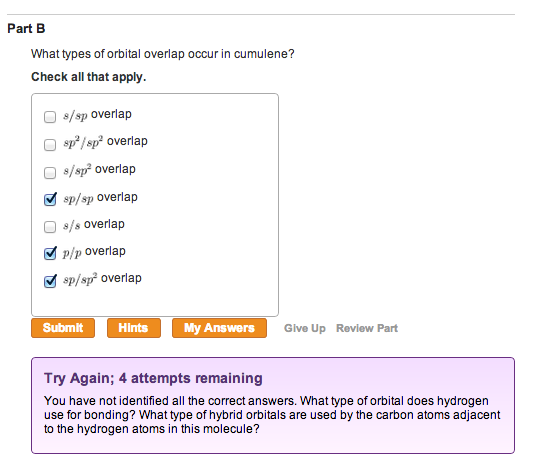
Figure 1 of 1 Cumulene What types of orbital overlap occur in cumulene? Check all that apply. S/SP overlap sp2/sp2 overlap s/sp2 overlap sp/sp overlap s/s overlap p/p overlap sp/sp2 overlap You have not identified all the correct answers. What type of orbital does hydrogen use for bonding? What type of hybrid orbitais are used by the carbon atoms adjacent to the hydrogen atoms in this molecule?

The correct answer and explanation is:
Cumulene is a unique hydrocarbon characterized by its alternating double bonds (C=C=C=C) along a linear chain. To address the questions, let’s analyze the orbital overlaps and hybridization involved in its bonding.
Correct Orbital Overlaps in Cumulene:
- s/sp overlap: Hydrogen atoms bond to the terminal carbon atoms through the overlap of the hydrogen 1s orbital with the sp hybrid orbital of the carbon atoms adjacent to hydrogen.
- sp/sp2 overlap: The terminal carbon atoms are sp hybridized, and they bond with sp² hybrid orbitals of the adjacent carbons.
- p/p overlap: The double bonds (C=C) consist of one sigma bond formed by sp²-sp² overlap and one pi bond formed by side-to-side overlap of unhybridized p orbitals.
Explanation of Orbital Hybridization:
- Hydrogen bonding: Hydrogen atoms use their 1s orbitals to form bonds. In cumulene, the hydrogen bonds to the terminal carbons, which are sp hybridized. The overlap is therefore s/sp.
- Hybridization of terminal carbon atoms: The terminal carbons are bonded to one hydrogen atom and one adjacent carbon atom. They use sp hybrid orbitals, with one sp orbital overlapping with hydrogen’s 1s orbital and the other overlapping with the sp² orbital of the adjacent carbon.
- Hybridization of internal carbon atoms: The internal carbons involved in double bonds are sp² hybridized. Each carbon atom forms:
- One sigma bond with another carbon via sp²/sp² overlap.
- One sigma bond with the next carbon (sp/sp² overlap).
- Pi bonds through p/p overlap for the double bonds.
Summary of Orbital Overlaps:
- s/sp overlap: Hydrogen-carbon bond.
- sp/sp2 overlap: Terminal carbon to adjacent internal carbon bond.
- p/p overlap: Formation of pi bonds in C=C double bonds.
Understanding these overlaps highlights the linear geometry and bonding structure of cumulene, with its alternating hybridization patterns between carbons.