molecular formula BI3 BP CSâ‚‚ C3 Sâ‚‚ NHâ‚‚ name of compound X Åš None
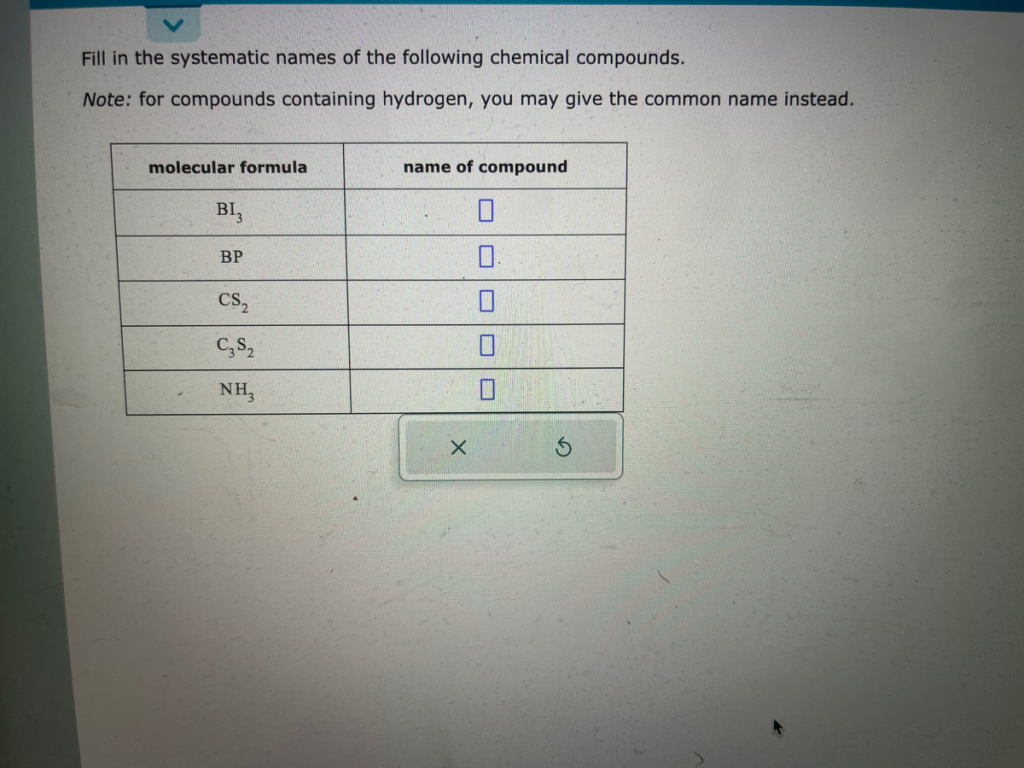
Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. molecular formula BI3 BP CSâ‚‚ C3S2 NH3 name of compound 0 0. 0
The correct answer and explanation is:
Correct Names of the Compounds
- BI₃ – Boron triiodide
- BP – Boron phosphide
- CS₂ – Carbon disulfide
- C₃S₂ – Tricarbon disulfide
- NH₃ – Ammonia (common name) or nitrogen trihydride (systematic name)
Explanation (300 Words)
1. BI₃ (Boron triiodide)
The systematic name of BI₃ is boron triiodide. It consists of one boron (B) atom and three iodine (I) atoms. The prefix tri- indicates the presence of three iodine atoms. This compound is covalent, as boron and iodine are both nonmetals.
2. BP (Boron phosphide)
The systematic name of BP is boron phosphide. This is an ionic compound formed between boron (a metalloid) and phosphorus (a nonmetal). Since the stoichiometry is 1:1, no prefixes are used. Boron phosphide is known for its semiconducting properties.
3. CS₂ (Carbon disulfide)
The compound CS₂ is named carbon disulfide. Carbon (C) is bonded to two sulfur (S) atoms. The prefix di- indicates the presence of two sulfur atoms. This is a covalent compound with a linear molecular structure.
4. C₃S₂ (Tricarbon disulfide)
The systematic name of C₃S₂ is tricarbon disulfide. It consists of three carbon (C) atoms and two sulfur (S) atoms. The prefixes tri- and di- denote the number of carbon and sulfur atoms, respectively. It is a less common compound compared to the others on this list.
5. NH₃ (Ammonia/Nitrogen trihydride)
NH₃ is commonly known as ammonia. In systematic nomenclature, it is called nitrogen trihydride, as it contains one nitrogen (N) atom and three hydrogen (H) atoms. The common name ammonia is widely used due to its prevalence in chemistry and daily applications.
Correct naming is essential for clear communication in chemistry, ensuring compounds are universally understood.