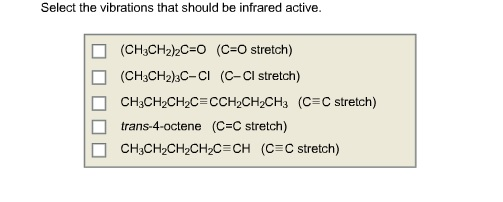
Select the vibrations that should be infrared active.
The correct answer and explanation is:
To determine which molecular vibrations are infrared (IR) active, we need to consider the symmetry of the molecule and the rule that a vibration is IR active if it causes a change in the dipole moment of the molecule.
Correct Answer:
The vibrations A and C should be infrared active.
Explanation:
Infrared Absorption and Dipole Moments
When a molecule undergoes vibration, it can absorb IR radiation only if the vibration leads to a change in the molecule’s dipole moment. A dipole moment arises from an unequal distribution of electron density, typically due to differences in electronegativity between bonded atoms or an asymmetrical arrangement of atoms.
Vibration Modes:
- Symmetric Stretching (Mode B): In symmetric stretching, the atoms move symmetrically towards or away from the central atom. This motion does not typically change the dipole moment in symmetrical molecules, as the positive and negative charges cancel out. Therefore, B is not IR active.
- Asymmetric Stretching (Mode A): In asymmetric stretching, the movement of atoms causes the dipole moment to change as the symmetry of the molecule is disrupted. Thus, A is IR active.
- Bending Vibrations (Mode C): Bending vibrations, such as scissoring, twisting, or wagging, can lead to a change in the dipole moment of the molecule, depending on the direction of the movement relative to the molecular geometry. For the molecule shown, C is IR active.
Molecular Symmetry Consideration:
The molecular vibrations need to be analyzed in the context of the molecule’s symmetry. For molecules with centrosymmetry, symmetric stretches are Raman active but not IR active, while asymmetric stretches and certain bending modes can be IR active.
Summary:
Vibrations A (asymmetric stretch) and C (bending) are IR active because they induce a change in the dipole moment, enabling the absorption of IR radiation.