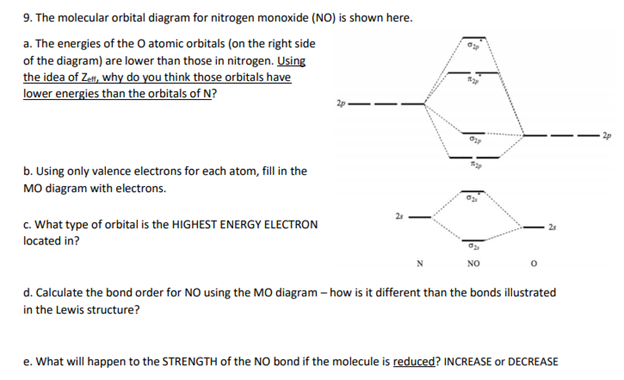
The molecular orbital diagram for nitrogen monoxide (NO) is shown here. a. The energies of the atomic orbitals (on the right side of the diagram) are lower than those in nitrogen. Using the idea of Zelf, why do you think those orbitals have lower energies than the orbitals of N? 2p b. Using only valence electrons for each atom, fill in the MO diagram with electrons. 2s – c. What type of orbital is the HIGHEST ENERGY ELECTRON located in? NO d. Calculate the bond order for NO using the MO diagram – how is it different than the bonds illustrated in the Lewis structure? e. What will happen to the STRENGTH of the NO bond if the molecule is reduced? INCREASE or DECREASE
The Correct Answer and Explanation is :
The molecular orbital (MO) diagram for nitrogen monoxide (NO) provides valuable insights into its electronic structure and bonding characteristics. Let’s address each part of the question in detail:
a. Why are the atomic orbitals of oxygen lower in energy than those of nitrogen?
Oxygen has a higher atomic number (Z = 8) compared to nitrogen (Z = 7), meaning it has an additional proton in its nucleus. This increased positive charge results in a greater effective nuclear charge (Z_eff), which is the net positive charge experienced by an electron in the atom. A higher Z_eff pulls electrons closer to the nucleus, reducing their energy levels. Consequently, the atomic orbitals of oxygen are lower in energy than those of nitrogen.
b. Filling the MO diagram with valence electrons:
Nitrogen has 5 valence electrons, and oxygen has 6, totaling 11 valence electrons for the NO molecule. According to molecular orbital theory, these electrons are distributed as follows:
- σ(2s) bonding orbital: 2 electrons
- σ*(2s) antibonding orbital: 2 electrons
- π(2p_x) and π(2p_y) bonding orbitals: 4 electrons (2 in each)
- σ(2p_z) bonding orbital: 2 electrons
- π*(2p_x) antibonding orbital: 1 electron
This configuration reflects the filling of molecular orbitals from lowest to highest energy, adhering to the Pauli exclusion principle and Hund’s rule.
c. Type of orbital for the highest energy electron:
The highest energy electron in NO occupies the π*(2p_x) antibonding orbital. This is the last orbital filled in the MO diagram for NO, containing a single unpaired electron.
d. Calculating the bond order and comparison with the Lewis structure:
Bond order is calculated using the formula:
[ \text{Bond order} = \frac{(\text{Number of bonding electrons}) – (\text{Number of antibonding electrons})}{2} ]
For NO:
- Bonding electrons: 2 (σ2s) + 4 (π2p_x and π2p_y) + 2 (σ2p_z) = 8
- Antibonding electrons: 2 (σ2s) + 1 (π2p_x) = 3
Thus, the bond order is:
[ \frac{8 – 3}{2} = 2.5 ]
In contrast, the Lewis structure of NO typically depicts a double bond between nitrogen and oxygen. The MO analysis reveals a bond order of 2.5, indicating a bond strength between a double and triple bond, which the Lewis structure does not capture.
e. Effect of reduction on the NO bond strength:
Reducing NO involves adding an electron, resulting in the NO⁻ anion. This additional electron would occupy the next available antibonding orbital, specifically the π*(2p_y) orbital. The bond order for NO⁻ would then be:
- Bonding electrons: 8
- Antibonding electrons: 4 (2 in σ2s and 2 in π2p_x and π*2p_y)
Calculating the new bond order:
[ \frac{8 – 4}{2} = 2.0 ]
Therefore, the bond order decreases from 2.5 in NO to 2.0 in NO⁻, indicating that the bond strength decreases upon reduction. This is because the addition of an electron to an antibonding orbital weakens the overall bond.
In summary, the MO diagram of NO provides a more nuanced understanding of its bonding characteristics than the Lewis structure, highlighting the molecule’s partial bond orders and the effects of electron addition or removal on bond strength.