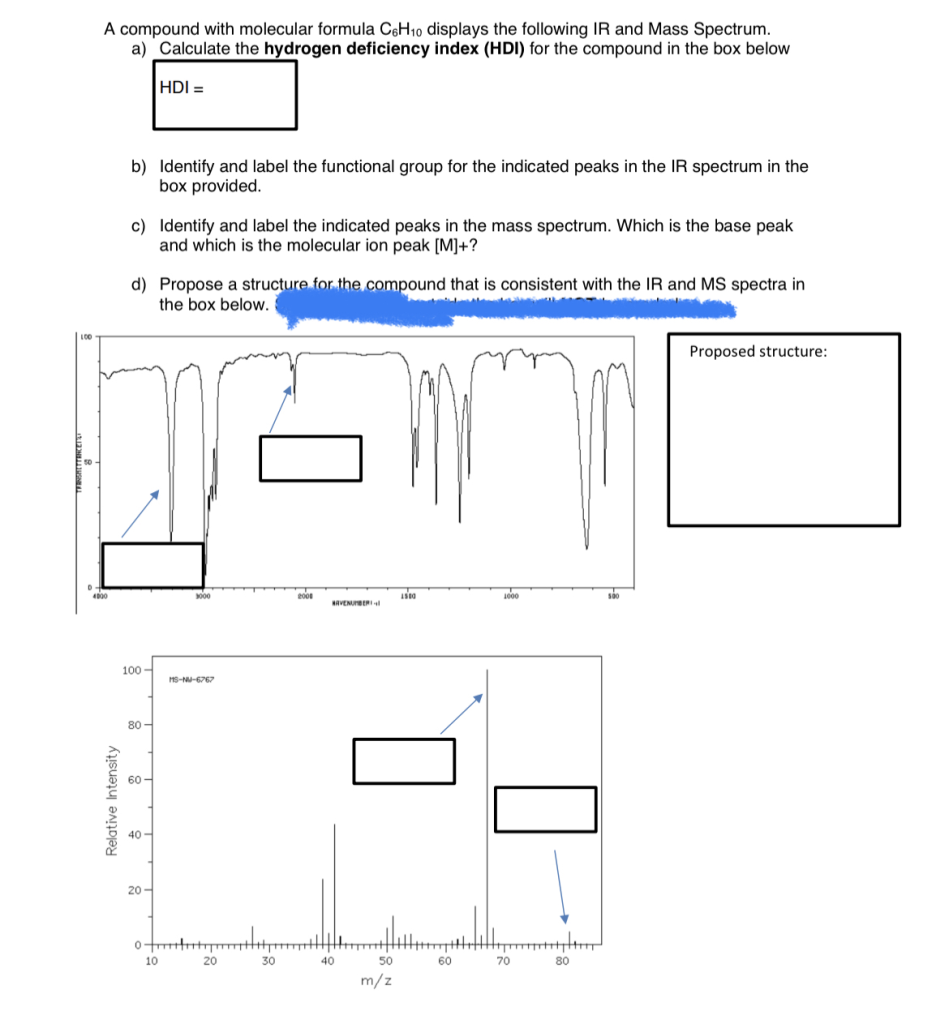
A compound with molecular formula C6H10 displays the following IR and Mass Spectrum. a) Calculate the hydrogen deficiency index (HDI) for the compound in the box below HDI = b) Identify and label the functional group for the indicated peaks in the IR spectrum in the box provided. c) Identify and label the indicated peaks in the mass spectrum. Which is the base peak and which is the molecular ion peak [M]+? d) Propose a structure for the compound that is consistent with the IR and MS spectra in the box below.
The Correct Answer and Explanation is :
To determine the structure of a compound with the molecular formula C₆H₁₀, we can analyze its hydrogen deficiency index (HDI), infrared (IR) spectrum, and mass spectrum.
a) Hydrogen Deficiency Index (HDI):
The HDI indicates the number of degrees of unsaturation (rings and/or multiple bonds) in a molecule. It is calculated using the formula:
HDI=2C+2−H2\text{HDI} = \frac{2C + 2 – H}{2}
For C₆H₁₀:
HDI=2(6)+2−102=12+2−102=42=2\text{HDI} = \frac{2(6) + 2 – 10}{2} = \frac{12 + 2 – 10}{2} = \frac{4}{2} = 2
Thus, the compound has an HDI of 2, indicating two degrees of unsaturation, which could be two double bonds, one triple bond, one ring plus one double bond, or two rings.
b) IR Spectrum Analysis:
Key absorption peaks in the IR spectrum help identify functional groups:
- ~3000 cm⁻¹: Indicates C–H stretching.
- ~1650 cm⁻¹: Corresponds to C=C stretching, suggesting the presence of an alkene.
- ~1450 cm⁻¹ and ~1375 cm⁻¹: Associated with C–H bending vibrations in methyl and methylene groups.
These absorptions are consistent with an alkene functional group.
c) Mass Spectrum Analysis:
In the mass spectrum:
- m/z 82: The molecular ion peak [M]⁺, confirming the molecular weight of 82 g/mol.
- m/z 67: A significant fragment, resulting from the loss of a methyl group (15 mass units) from the molecular ion.
- m/z 54: Another fragment, indicating further cleavage, possibly from the loss of an ethyl group (28 mass units) from the molecular ion.
- Base peak at m/z 67: The most intense peak, representing the most stable and abundant fragment ion.
These fragmentation patterns are characteristic of cyclohexene, where the loss of alkyl groups leads to stable carbocation fragments.
d) Proposed Structure:
Considering the HDI of 2, the presence of a C=C bond (alkene) from the IR spectrum, and the fragmentation pattern in the mass spectrum, the compound is likely cyclohexene.
Cyclohexene has one ring and one double bond, satisfying the HDI of 2. The IR absorptions align with those expected for cyclohexene citeturn0search1, and the mass spectrum fragmentation pattern matches that of cyclohexene citeturn0search0.
In summary, the compound with molecular formula C₆H₁₀ is identified as cyclohexene, based on the calculated HDI, IR spectral data indicating an alkene, and mass spectral fragmentation patterns consistent with known data for cyclohexene.