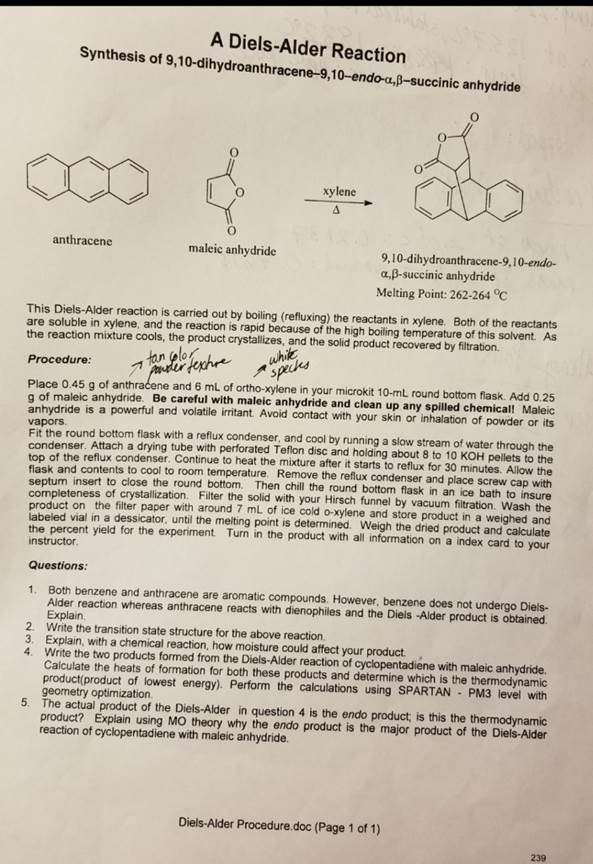
A Deils – Alder Reaction, Synthesis of 9, 10-dihydroanthracene-9,10-endo-alpha, Beta-succinic anhydride
The Correct Answer and Explanation is :
The Diels-Alder reaction between anthracene and maleic anhydride yields 9,10-dihydroanthracene-9,10-endo-α,β-succinic anhydride, a compound with significant applications in organic synthesis.
Mechanism:
In this reaction, anthracene serves as the diene, and maleic anhydride acts as the dienophile. The process involves a concerted [4+2] cycloaddition, where four π-electrons from the diene and two π-electrons from the dienophile form a six-membered ring. This reaction proceeds through the s-cis conformation of the diene, facilitating the formation of the cyclohexene ring. The product is characterized by the addition of the maleic anhydride across the central ring of anthracene, resulting in a bicyclic structure with fused rings.
Experimental Procedure:
- Reagents: Combine 4 g of anthracene and 2.2 g of maleic anhydride in a 250 mL round-bottom flask.
- Solvent: Add 50 mL of dry xylene to the flask.
- Reflux: Attach a reflux condenser and heat the mixture to reflux for 30-35 minutes.
- Purification: If the solution is colored, add 1 g of activated charcoal and reflux for an additional 10-15 minutes. Filter the hot solution through a Buchner funnel.
- Crystallization: Allow the filtrate to cool to room temperature, then place it in an ice bath to promote crystallization.
- Isolation: Collect the white crystalline product by filtration and dry it.
The theoretical yield of 9,10-dihydroanthracene-9,10-endo-α,β-succinic anhydride is calculated based on the molar masses of the reactants and the product. For instance, using 4 g of anthracene (molar mass 178 g/mol) and 2.2 g of maleic anhydride (molar mass 98 g/mol), the theoretical yield can be determined by stoichiometric calculations.
Characterization:
The synthesized product can be characterized by its melting point, which typically ranges from 256°C to 258°C. Infrared (IR) spectroscopy can confirm the presence of functional groups such as C=O (carbonyl) and C–O (ether), which are indicative of the succinic anhydride moiety. Additionally, nuclear magnetic resonance (NMR) spectroscopy can provide detailed information about the molecular structure, confirming the incorporation of the maleic anhydride into the anthracene framework.
This Diels-Alder reaction exemplifies a straightforward method for synthesizing fused bicyclic compounds, demonstrating the utility of pericyclic reactions in organic synthesis.