Write a complete chemical formula, including physical state symbol, for each element listed below in its standard state. The first row of the table has been completed for you.
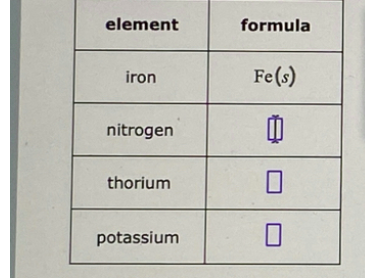
The Correct Answer and Explanation is :
In chemistry, the standard state of an element refers to its most stable physical form at 25°C and 1 bar pressure. This standardization is crucial for thermodynamic calculations, as it provides a consistent reference point.
For most elements, the standard state is solid. However, there are exceptions:
- Liquids: Bromine (Br₂) and mercury (Hg) are liquids at standard conditions.
- Gases: The noble gases (He, Ne, Ar, Kr, Xe, Rn), along with hydrogen (H₂), nitrogen (N₂), oxygen (O₂), fluorine (F₂), and chlorine (Cl₂), are gases at standard conditions.
Understanding the standard state of elements is essential for calculating standard enthalpies of formation, which are used to determine the heat change during chemical reactions. By defining a standard state, chemists can compare the thermodynamic properties of different substances under consistent conditions.
For example, the standard state of carbon is graphite, not diamond, because graphite is the most stable allotrope at standard conditions. Similarly, the standard state of phosphorus is white phosphorus (P₄), even though red phosphorus is more stable thermodynamically.
In summary, the standard state of an element is its most stable physical form at 25°C and 1 bar pressure, and this standardization is vital for consistent thermodynamic calculations in chemistry.