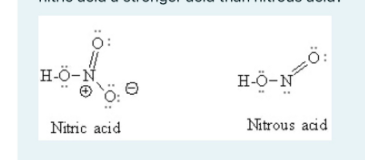
Nitric acid, HNO3, is a strong acid while nitrous acid, HNO2, is a weak acid. Why is nitric acid a stronger acid than nitrous acid? 0: -N H-O-N Nitric acid Nitrous acid Select one: A. Nitrogen has a larger atomic radius than oxygen B. Nitrate ion (NO3) has greater resonance stability than nitrite ion (NO2) OC. Nitrous acid exhibits greater steric hindrance to loss of a proton than nitric acid OD. Nitrogen is more electronegative than oxygen
The Correct Answer and Explanation is :
The correct answer is B. Nitrate ion (NO₃⁻) has greater resonance stability than nitrite ion (NO₂⁻).
Explanation:
The strength of an acid is determined by how readily it donates a proton (H⁺). The conjugate base (the ion that remains after proton donation) plays a crucial role in determining the acid strength. A more stable conjugate base results in a stronger acid because the proton is more likely to dissociate.
Resonance Stability:
- Nitric Acid (HNO₃): When nitric acid dissociates, it forms the nitrate ion (NO₃⁻). The nitrate ion has three resonance structures, meaning the negative charge is delocalized over three oxygen atoms. This delocalization significantly stabilizes the nitrate ion, making it less likely to recombine with a proton (H⁺), and thus enhances the acid strength of HNO₃.
- Nitrous Acid (HNO₂): In contrast, the nitrite ion (NO₂⁻), which is the conjugate base of nitrous acid, has only two resonance structures, meaning the negative charge is less delocalized compared to the nitrate ion. This results in a less stable conjugate base and thus a weaker acid (HNO₂) compared to nitric acid (HNO₃).
Other Options:
- A. Nitrogen has a larger atomic radius than oxygen: This statement is incorrect because the atomic radius of nitrogen is smaller than that of oxygen, and atomic radius alone does not significantly affect acid strength in this context.
- C. Nitrous acid exhibits greater steric hindrance to the loss of a proton than nitric acid: This is also not the main reason for the difference in acid strength. Both acids can lose a proton without significant steric hindrance; it’s the resonance stability of their conjugate bases that is more important.
- D. Nitrogen is more electronegative than oxygen: This is incorrect because oxygen is more electronegative than nitrogen. The difference in electronegativity between nitrogen and oxygen does not directly explain the stronger acidity of nitric acid.
Thus, the stronger acidity of nitric acid is primarily due to the greater resonance stability of the nitrate ion compared to the nitrite ion.