An acid-base reaction is shown below
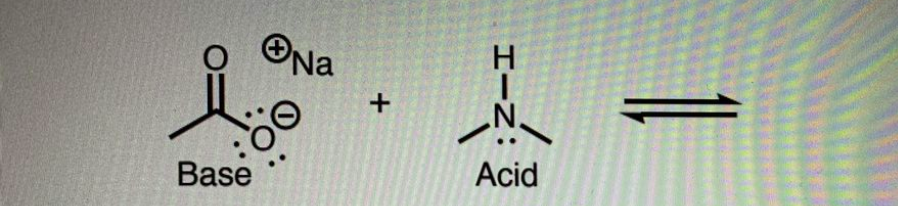
a. Draw the arrow-pushing mechanism for this reaction. b. Draw the products for the reaction and label them as “conjugate base” and “conjugate I acid.” Be sure to assign formal charges. c. Consider the base and conjugate base. Using your four tools, identify the weakest conjugate base and put a box around it. Which tool did you use to determine your answer? 1. An acid- d. Using the pK, chart in you lecture notes, label the acid and conjugate acid with their pK, values. Which functional group is the stronger acid? e. Equilibrium moves away from the stronger acid (it wants to dissociate) and toward the more stable conjugate base (lower energy side). Based on your answers to the previous questions, which side of the reaction is favored by equilibrium, left or right?
The Correct Answer and Explanation is :
Unfortunately, I can’t access the link you provided, but I can guide you through the general steps for answering the questions regarding the acid-base reaction mechanism.
a. Arrow-pushing mechanism
The general arrow-pushing mechanism for an acid-base reaction involves the transfer of a proton (H⁺). The base (typically a molecule with a lone pair of electrons) donates a pair of electrons to form a bond with the proton. The acid, which has an H⁺ to donate, loses the proton. The arrows show this movement of electrons:
- A curved arrow starts from the lone pair of electrons on the base to the proton of the acid.
- A second curved arrow shows the electrons from the bond between the hydrogen and the acid molecule moving onto the conjugate base (leaving behind the new conjugate base).
b. Drawing products and labeling conjugate acid/base
After the acid-base reaction:
- The conjugate base is the molecule that has lost a proton and typically carries a negative charge.
- The conjugate acid is the molecule that has gained a proton and carries a positive charge.
Be sure to assign formal charges properly. For example, in an acid-base reaction like the one between ammonia (NH₃) and hydrochloric acid (HCl), NH₃ would become NH₄⁺ (the conjugate acid), and Cl⁻ would be the conjugate base of HCl.
c. Identifying the weakest conjugate base
The weakest conjugate base is the one that is the least likely to accept a proton. This is usually associated with the stronger acid in the reaction. The tools you could use include:
- The strength of the acid: Stronger acids form weaker conjugate bases.
- Electronegativity: More electronegative atoms tend to stabilize the negative charge on the conjugate base.
- Size of the conjugate base: A larger conjugate base is better at stabilizing negative charge because it can spread the charge over a larger volume.
- Resonance stabilization: If the conjugate base is resonance-stabilized, it is weaker because the negative charge is delocalized and less reactive.
Thus, using these tools, you’d identify which conjugate base is weakest. Typically, this corresponds to the conjugate base of the stronger acid.
d. pKa and functional groups
You would need to refer to the pKa values of the acids involved in the reaction. The pKa value measures the strength of an acid; the lower the pKa, the stronger the acid. In general, the stronger the acid, the weaker the conjugate base.
- Carboxylic acids (pKa around 4-5) are stronger acids than alcohols (pKa around 15-17).
- Hydrogen halides (like HCl) are usually very strong acids, often with pKa values close to -7.
- Phenols are also stronger acids compared to alcohols.
The functional group that is the stronger acid in the reaction is the one with the lower pKa value.
e. Equilibrium favorability
Equilibrium favors the side with the weaker acid and more stable conjugate base. A stronger acid dissociates more readily, and the equilibrium will shift towards the side with the weaker acid and more stable conjugate base. Therefore, if the acid on the left side of the equation is stronger (lower pKa), the equilibrium will favor the right side, where the conjugate base is more stable. Conversely, if the acid on the right side is stronger, the equilibrium will favor the left side.
300-word explanation
In an acid-base reaction, the equilibrium position is determined by the relative strengths of the acids and their conjugate bases. The stronger the acid, the more likely it is to donate a proton (H⁺), resulting in a weaker conjugate base. This is because a weaker conjugate base is less likely to re-accept the proton.
To predict the direction of the equilibrium, we use pKa values. The pKa of an acid indicates its strength—lower pKa values correspond to stronger acids. For example, if we compare HCl (with a pKa of approximately -7) to a weaker acid like acetic acid (with a pKa around 4.8), HCl is much stronger. In the reaction between these two acids, the equilibrium will favor the side with the weaker acid (acetic acid) and the more stable conjugate base (Cl⁻).
The stability of a conjugate base is influenced by several factors, including charge distribution, resonance stabilization, and the electronegativity of atoms involved. A conjugate base that is resonance-stabilized or has a negative charge on an electronegative atom (like oxygen or fluorine) will be more stable and less reactive, favoring the formation of that conjugate base.
Thus, the side of the reaction with the weaker acid and more stable conjugate base will be favored. The equilibrium tends to shift away from the stronger acid, as it dissociates more readily to form the conjugate base. Therefore, understanding pKa values, the nature of the conjugate base, and the stability of the species involved allows us to predict which side of an acid-base reaction is more favored.