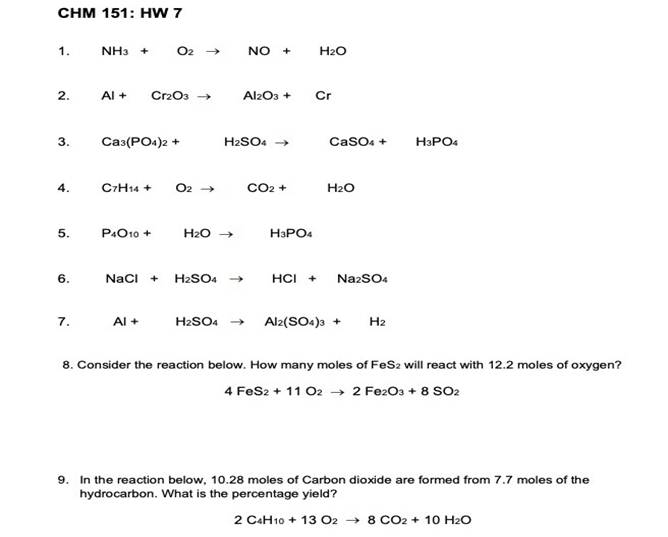
CHM 151: HW 7 1. NH O2 NO H₂O 2. Al+ Cr2O3 Al2O3+ Cr 3. Ca3(PO4)2 + H2SO4 CaSO+ H3PO4 4. C7H14+ O2 CO2 + H2O 5. P4O10+ H₂O H3PO4 6. NaCl + H2SO4 HCI Na2SO4 7. Al+ H2SO4 Al2(SO4)3 H 8. Consider the reaction below. How many moles of FeS: will react with 12.2 moles of oxygen? 4 FeS2 + 11 02 2 Fe2O3 + 8 SO2 9. In the reaction below, 10.28 moles of Carbon dioxide are formed from 7.7 moles of the hydrocarbon. What is the percentage yield? 2 C4H10 13 028 CO2 10 H₂O
The Correct Answer and Explanation is :
To help with your HW, I’ll break down each question and provide the relevant steps to find the answers.
1. NH₂O + O₂ → NO + H₂O
This is a redox reaction where nitrogen in NH₂O is oxidized to NO, and oxygen is reduced. To balance this, you’d need to ensure both mass and charge balance. The molecular weights of NH₂O, O₂, NO, and H₂O will help ensure that the atoms are conserved.
2. Al + Cr₂O₃ → Al₂O₃ + Cr
This is a redox reaction between aluminum and chromium oxide. Aluminum displaces chromium because it is more reactive. The stoichiometric coefficients should balance to ensure the conservation of atoms. The reaction would look like this after balancing:
[
6Al + Cr₂O₃ \rightarrow 2Al₂O₃ + 2Cr
]
3. Ca₃(PO₄)₂ + H₂SO₄ → CaSO₄ + H₃PO₄
This is an acid-base reaction where calcium phosphate reacts with sulfuric acid. The products are calcium sulfate and phosphoric acid. The balanced equation will be:
[
Ca₃(PO₄)₂ + 3H₂SO₄ → 3CaSO₄ + 2H₃PO₄
]
4. C₇H₁₄ + O₂ → CO₂ + H₂O
This is a combustion reaction where the hydrocarbon (heptane) reacts with oxygen. The balanced equation will be:
[
2C₇H₁₄ + 21O₂ → 14CO₂ + 14H₂O
]
This ensures the conservation of carbon, hydrogen, and oxygen atoms.
5. P₄O₁₀ + H₂O → H₃PO₄
This is a synthesis reaction where phosphorus pentoxide reacts with water to form phosphoric acid. The balanced equation is:
[
P₄O₁₀ + 6H₂O → 4H₃PO₄
]
6. NaCl + H₂SO₄ → HCl + Na₂SO₄
This is an acid-base reaction where sodium chloride reacts with sulfuric acid. The balanced equation is:
[
2NaCl + H₂SO₄ → 2HCl + Na₂SO₄
]
7. Al + H₂SO₄ → Al₂(SO₄)₃ + H₂
This is another redox reaction between aluminum and sulfuric acid. The balanced equation is:
[
2Al + 3H₂SO₄ → Al₂(SO₄)₃ + 3H₂
]
8. How many moles of FeS₂ will react with 12.2 moles of oxygen?
The balanced reaction is:
[
4FeS₂ + 11O₂ → 2Fe₂O₃ + 8SO₂
]
We are asked to determine how many moles of FeS₂ will react with 12.2 moles of O₂. Using stoichiometry, we know:
[
4 \text{ moles of FeS₂} : 11 \text{ moles of O₂}
]
So, for 12.2 moles of O₂, the moles of FeS₂ required will be:
[
\frac{4 \text{ moles of FeS₂}}{11 \text{ moles of O₂}} \times 12.2 \text{ moles of O₂} = 4.44 \text{ moles of FeS₂}
]
So, 4.44 moles of FeS₂ will react with 12.2 moles of oxygen.
9. Percentage yield of CO₂ from 7.7 moles of C₄H₁₀
The balanced equation is:
[
2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
]
From the stoichiometry, 2 moles of butane produce 8 moles of CO₂. So, 7.7 moles of C₄H₁₀ will produce:
[
7.7 \text{ moles of C₄H₁₀} \times \frac{8 \text{ moles of CO₂}}{2 \text{ moles of C₄H₁₀}} = 30.8 \text{ moles of CO₂}
]
Now, if the actual yield of CO₂ is 10.28 moles, the percentage yield will be:
[
\frac{10.28}{30.8} \times 100 = 33.4\%
]
So the percentage yield is 33.4%.
These are the balanced reactions and solutions to your questions. If you need any further details or clarification, feel free to ask!