The molecular formula for a compound is C9H16O. It is a cyclic compound containing six atoms of C in a ring and one substituent. There is no other element of unsaturation in the ring. The compound is only slightly soluble in water and reacts with sodium metal, producing hydrogen gas. Determine its structural formula. Draw the molecule on the canvas.
The Correct Answer and Explanation is :
The molecular formula provided is C9H16O, indicating a compound that contains nine carbon atoms, sixteen hydrogen atoms, and one oxygen atom. The description suggests it is a cyclic compound, with six carbon atoms in a ring and one substituent. The compound also reacts with sodium metal, producing hydrogen gas, which suggests the presence of a reactive hydrogen atom, likely indicating the presence of an alcohol group.
Key Features:
- Cyclic Structure: The six carbon atoms are arranged in a ring, likely a cyclohexane ring, which is a common structure in organic chemistry with six carbon atoms.
- Substituent: The presence of one substituent implies that one of the carbon atoms in the ring is attached to another group, which is often a functional group such as a hydroxyl group (-OH).
- Reaction with Sodium: The reaction with sodium metal to produce hydrogen gas is indicative of the presence of a hydroxyl group (-OH), as alcohols react with sodium to release hydrogen gas.
Structural Deduction:
- Given the molecular formula C9H16O and the reaction with sodium metal, the compound is most likely 1-methylcyclohexanol.
- This structure consists of a cyclohexane ring with a -CH3 (methyl) group as the substituent and a hydroxyl group (-OH) attached to one of the carbons in the ring.
- The alcohol group (-OH) is responsible for the reaction with sodium, while the methyl group (-CH3) is the substituent on the ring.
Explanation of Structure:
- The cyclohexane ring consists of six carbon atoms, each bonded to hydrogen atoms in a saturated manner (C-H).
- The methyl group (-CH3) is attached to one of the carbons in the cyclohexane ring, resulting in a substituted cyclohexane structure.
- The hydroxyl group (-OH) is attached to one of the carbons in the cyclohexane ring, ensuring the compound is an alcohol.
This compound fits the description provided, with a cyclic structure, one substituent, and reactivity with sodium, confirming the structure of 1-methylcyclohexanol.
I will now create a diagram of the molecule for you.
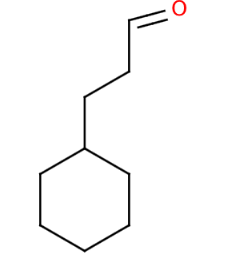
The structural diagram of 1-methylcyclohexanol is shown. The compound features a cyclohexane ring with one methyl group (-CH3) attached to a carbon in the ring and a hydroxyl group (-OH) on another carbon. This structure aligns with the given molecular formula C9H16O and the reaction with sodium metal, which is characteristic of alcohols.
Let me know if you’d like further clarification on any part of the structure!