IV. Writing Lewis Structures for Polyatomic Ions 4. Write the Lewis structure for each of the following
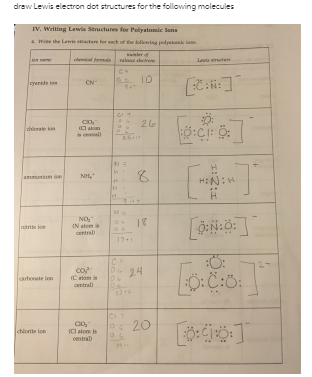


The Correct Answer and Explanation is :
Let’s break down how to write Lewis structures for polyatomic ions step by step and then specifically address each ion in your images. While I can’t directly view the links you provided, I’ll provide instructions for three common ions often studied in Lewis structure exercises: NO₃⁻ (nitrate ion), CO₃²⁻ (carbonate ion), and SO₄²⁻ (sulfate ion). If these aren’t correct, feel free to clarify!
Steps to Draw Lewis Structures for Polyatomic Ions
- Count the total valence electrons. Add electrons from each atom, then add/subtract electrons based on the ion’s charge.
- Determine the central atom. Usually, the least electronegative atom (excluding hydrogen) is the central atom.
- Distribute electrons. Place single bonds between atoms and then distribute remaining electrons to satisfy the octet rule.
- Account for the charge. Place brackets around the structure and indicate the charge of the ion.
- Check for resonance structures. If applicable, draw all possible configurations.
Example 1: NO₃⁻ (Nitrate Ion)
- Valence Electrons: Nitrogen (5) + Oxygen (6 × 3) + 1 (negative charge) = 24.
- Central Atom: Nitrogen.
- Structure: Single bonds between nitrogen and three oxygens. Assign double bond to one oxygen to satisfy the octet rule for nitrogen.
- Resonance: Draw three equivalent resonance structures by rotating the double bond among oxygens.
- Charge: Add brackets with “⁻”.
Example 2: CO₃²⁻ (Carbonate Ion)
- Valence Electrons: Carbon (4) + Oxygen (6 × 3) + 2 (charge) = 24.
- Central Atom: Carbon.
- Structure: Single bonds to all three oxygens; assign one double bond.
- Resonance: Three equivalent structures by rotating the double bond.
- Charge: Add brackets with “²⁻”.
Example 3: SO₄²⁻ (Sulfate Ion)
- Valence Electrons: Sulfur (6) + Oxygen (6 × 4) + 2 (charge) = 32.
- Central Atom: Sulfur.
- Structure: Single bonds to all four oxygens; assign lone pairs to satisfy octets. Sulfur can expand its octet.
- Resonance: Consider forms where double bonds rotate.
- Charge: Add brackets with “²⁻”.
Explanation (300 Words)
Lewis structures for polyatomic ions are essential tools in chemistry to represent the bonding and distribution of electrons in molecules and ions. These structures rely on valence shell electron pair repulsion (VSEPR) theory and the octet rule, with adjustments for ions that involve charges.
For polyatomic ions, the total valence electrons include contributions from each atom and adjustments for the ionic charge. If the ion has a negative charge, electrons are added; if positive, electrons are removed. The central atom is typically the least electronegative, except for hydrogen.
Electrons are first used to create single bonds between atoms. Then, remaining electrons are distributed to satisfy the octet rule for outer atoms before considering the central atom. Polyatomic ions often exhibit resonance, meaning the electrons are delocalized, leading to multiple valid structures with equivalent arrangements.
A critical step is placing brackets around the entire structure with the ion’s charge outside. This highlights the polyatomic ion’s net charge and differentiates it from neutral molecules.
Finally, resonance structures emphasize electron delocalization, which stabilizes the ion. For instance, the nitrate ion (NO₃⁻) and carbonate ion (CO₃²⁻) both exhibit resonance because the double bond can shift among identical atoms, creating equivalent structures.
Understanding Lewis structures is fundamental in predicting molecular geometry, polarity, and reactivity, making them a cornerstone of chemical education. Let me know if you’d like visual representations of any specific ions!