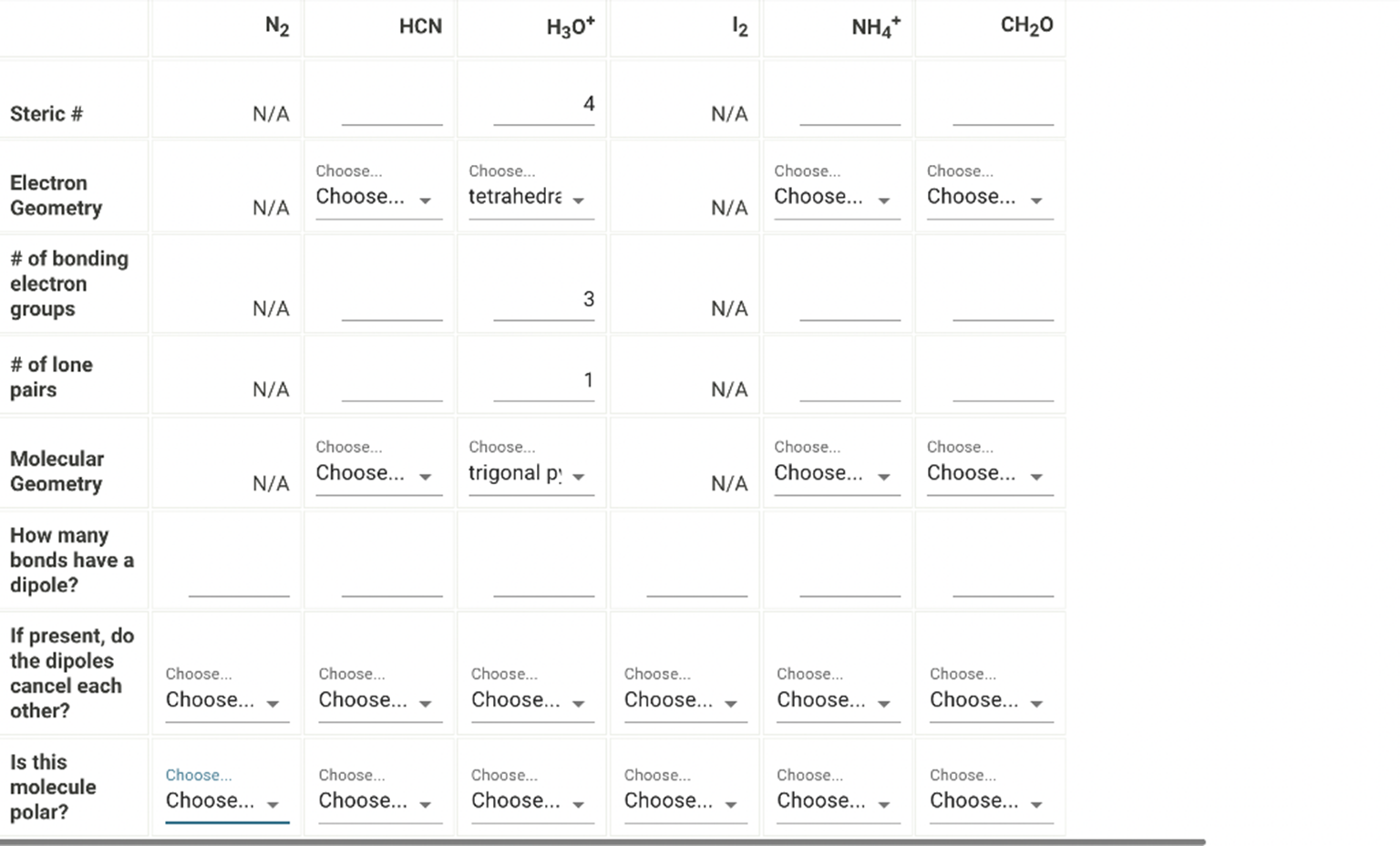
N2 HCN H30+ 12 NH4+ CH20 4 Steric # N/A N/A Electron Geometry Choose.. Choose… – Choose.. tetrahedra Choose Choose… Choose Choose… N/A N/A # of bonding electron groups 3 N/A N/A # of lone pairs N/A 1 N/A Choose… Molecular Geometry Choose.. Choose… – trigonalp! – Choose. Choose… Choose Choose… – N/A N/A How many bonds have a dipole? If present, do the dipoles cancel each other? Choose.. Choose… – Choose. Choose… – Choose… Choose… – Choose.. Choose… Choose Choose… Choose Choose… – Is this molecule polar? Choose Choose… – Choose. Choose… – Choose Choose… – Choose… Choose… Choose Choose… Choose Choose…
The Correct Answer and Explanation is :
The information provided is somewhat incomplete or difficult to interpret, but I can still help you break down the molecule and answer the relevant questions. It appears to involve several molecules or ions, so let’s clarify and address each component step by step.
Molecules/Compounds in the Question:
- N₂ (Nitrogen molecule)
- HCN (Hydrogen Cyanide)
- H₃O⁺ (Hydronium ion)
- NH₄⁺ (Ammonium ion)
- CH₂O (Formaldehyde)
Step-by-Step Explanation:
- Electron Geometry:
- N₂: Linear, because it’s a simple diatomic molecule (N≡N).
- HCN: Linear, as it consists of a central carbon atom triple-bonded to nitrogen and single-bonded to hydrogen.
- H₃O⁺: Tetrahedral, with one oxygen bonded to three hydrogens, and the oxygen atom carries a lone pair.
- NH₄⁺: Tetrahedral, as the nitrogen is bonded to four hydrogens with no lone pairs.
- CH₂O: Trigonal planar, with carbon in the center and double-bonded to oxygen while single-bonded to two hydrogens.
- Bonding Electron Groups & Lone Pairs:
- N₂: There are two bonding electron groups (triple bond between N atoms), no lone pairs.
- HCN: Three bonding electron groups (C-H and C≡N), no lone pairs on C or N.
- H₃O⁺: Three bonding electron groups (O-H) and one lone pair on the oxygen atom.
- NH₄⁺: Four bonding electron groups, no lone pairs on nitrogen.
- CH₂O: Three bonding electron groups (C-H and C=O), no lone pairs on carbon.
- Molecular Geometry:
- N₂: Linear.
- HCN: Linear.
- H₃O⁺: Tetrahedral.
- NH₄⁺: Tetrahedral.
- CH₂O: Trigonal planar.
- Dipoles:
- N₂: No dipole, as it’s a homonuclear diatomic molecule.
- HCN: Yes, HCN has a dipole because of the difference in electronegativity between carbon and nitrogen.
- H₃O⁺: Yes, the H-O bond has a dipole. The hydronium ion is polar due to the lone pair on oxygen.
- NH₄⁺: No dipole, because the tetrahedral geometry is symmetrical, and the bond dipoles cancel.
- CH₂O: Yes, CH₂O has a dipole moment because the oxygen is more electronegative than carbon and hydrogen.
- Polarity:
- N₂: Nonpolar, as the dipoles cancel out.
- HCN: Polar, as the dipoles don’t cancel.
- H₃O⁺: Polar, due to the lone pair on oxygen and the distribution of electron density.
- NH₄⁺: Nonpolar, due to symmetry.
- CH₂O: Polar, as the dipoles don’t cancel.
Conclusion:
Based on the molecular structures, we can conclude that some of the molecules or ions are polar (like HCN, H₃O⁺, and CH₂O), while others are nonpolar (like N₂ and NH₄⁺). Dipoles exist in molecules that have significant electronegativity differences between bonded atoms, such as in HCN, H₃O⁺, and CH₂O.