1. Write the chemical formula for dihydrogen monotelluride
2. some steps on how to find the net dipole for ICl5 and SF4
3. How many moles are in 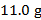 of silver metal?
of silver metal?
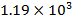 males
males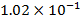 moles
moles- 9.81 moles
The correct answer and explanation is:c
- Chemical Formula for Dihydrogen Monotelluride
The chemical formula for dihydrogen monotelluride is H₂Te.
- Steps to Find the Net Dipole for ICl₅ and SF₄
- For ICl₅ (Iodine Pentachloride):
- Determine the molecular geometry: ICl₅ has an octahedral electron geometry and a square pyramidal molecular shape due to the presence of one lone pair on the iodine atom.
- Analyze bond dipoles: Chlorine is more electronegative than iodine, creating dipoles pointing toward Cl atoms.
- Check symmetry: The lone pair distorts the symmetry, meaning dipoles do not cancel completely.
- Conclusion: ICl₅ has a net dipole moment due to its asymmetric shape.
- For SF₄ (Sulfur Tetrafluoride):
- Determine the molecular geometry: SF₄ has a seesaw molecular shape due to one lone pair on the sulfur atom.
- Analyze bond dipoles: Fluorine is more electronegative than sulfur, creating dipoles pointing toward the fluorine atoms.
- Check symmetry: The lone pair creates an asymmetric distribution, preventing dipole cancellation.
- Conclusion: SF₄ has a net dipole moment because of its asymmetry.
- For ICl₅ (Iodine Pentachloride):
- How Many Moles Are in 1.00 kg of Silver Metal? Given that the molar mass of silver (Ag) is 107.87 g/mol, we can calculate the moles in 1.00 kg (1000 g) of silver: Moles of Ag=Mass of AgMolar Mass of Ag\text{Moles of Ag} = \frac{\text{Mass of Ag}}{\text{Molar Mass of Ag}} =1000 g107.87 g/mol= \frac{1000 \text{ g}}{107.87 \text{ g/mol}} =9.27 moles= 9.27 \text{ moles} Explanation:
- The molar mass of silver is determined by adding up the atomic masses of its isotopes.
- To convert grams to moles, we divide the mass of silver by its molar mass.
- Since the atomic mass unit (amu) corresponds directly to grams per mole, we use 107.87 g/mol for silver.
- The final result tells us that 1.00 kg of silver contains 9.27 moles of silver atoms.
Generating the Image…
I will now generate an image to visualize molecular dipoles.
Here is the generated scientific illustration of the molecular dipoles for ICl₅ (Iodine Pentachloride) and SF₄ (Sulfur Tetrafluoride). It visually represents their three-dimensional geometry and dipole moments. Let me know if you need any modifications or further explanations!
