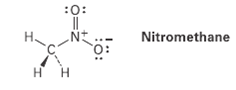
Nitromethane has the structure indicated. Explain why it must have formal charges on N andO.
The Correct Answer and Explanation is :

Nitromethane (CH₃NO₂) consists of a methyl group (–CH₃) bonded to a nitro group (–NO₂). The nitro group comprises a nitrogen atom bonded to two oxygen atoms, one of which is doubly bonded, and the other singly bonded with a negative formal charge.
Formal Charges in Nitromethane:
- Nitrogen (N) Atom:
- Valence Electrons: Nitrogen, being in Group 15 of the periodic table, has five valence electrons.
- Bonding Electrons: In the nitro group, nitrogen forms one double bond and one single bond, sharing a total of four electrons.
- Non-Bonding Electrons: Nitrogen has no lone pairs in this structure.
- Formal Charge Calculation: Formal Charge (FC) = Valence Electrons – (Non-Bonding Electrons + ½ Bonding Electrons)
- FC(N) = 5 – (0 + ½ × 4) = 5 – 2 = +1
- Conclusion: The nitrogen atom carries a formal charge of +1.
- Oxygen (O) Atoms:
- Valence Electrons: Each oxygen atom, being in Group 16, has six valence electrons.
- Bonding Electrons: One oxygen forms a double bond with nitrogen (sharing four electrons), and the other forms a single bond (sharing two electrons).
- Non-Bonding Electrons: The singly bonded oxygen has three lone pairs (six electrons), while the doubly bonded oxygen has two lone pairs (four electrons).
- Formal Charge Calculation for Singly Bonded Oxygen:
- FC(O₁) = 6 – (6 + ½ × 2) = 6 – 7 = –1
- Formal Charge Calculation for Doubly Bonded Oxygen:
- FC(O₂) = 6 – (4 + ½ × 4) = 6 – 6 = 0
- Conclusion: The singly bonded oxygen carries a formal charge of –1, and the doubly bonded oxygen has no formal charge.
Resonance Structures:
Nitromethane exhibits resonance, meaning the actual structure is a hybrid of multiple contributing structures. The resonance involves the shifting of electrons between nitrogen and oxygen atoms, leading to different formal charge distributions. These resonance forms stabilize the molecule by delocalizing the charges.