The monomers of lipids are called fatty acids. Draw the lipid monomer a fatty acid, labeling the hydrocarbon chain and the carboxyl end group. Be sure to show ALL of the atoms within the molecule (there are several different fatty acids in living things including oleic acid and stearic acid, each with a unique molecular formula).
The Correct Answer and Explanation is :
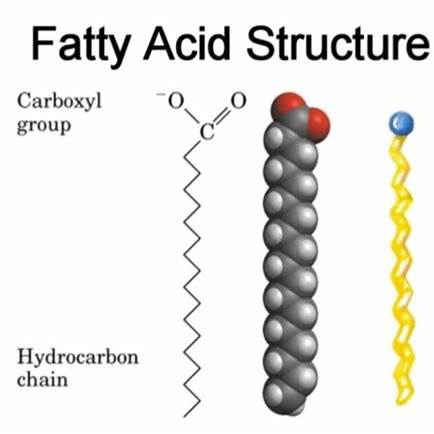
Fatty acids are fundamental monomers of lipids, consisting of a hydrocarbon chain attached to a carboxyl group (–COOH). The hydrocarbon chain varies in length, typically containing an even number of carbon atoms ranging from 4 to 36, with 12 to 18 being most common.
Structure of a Fatty Acid:
- Carboxyl Group (–COOH): This functional group comprises a carbonyl group (C=O) and a hydroxyl group (–OH) attached to the same carbon atom. It imparts acidic properties to the molecule.
- Hydrocarbon Chain: A long chain of carbon atoms bonded to hydrogen atoms. The chain can be saturated (no double bonds between carbon atoms) or unsaturated (one or more double bonds present).
Example Structures:
- Stearic Acid (C18H36O2): A saturated fatty acid with a straight hydrocarbon chain of 18 carbon atoms, each bonded to hydrogen atoms, ending with a carboxyl group.
- Oleic Acid (C18H34O2): An unsaturated fatty acid with a similar hydrocarbon chain but containing one double bond, typically in the cis configuration, causing a bend in the chain.
Explanation:
The carboxyl group at one end of the fatty acid makes it polar, allowing it to interact with water molecules. In contrast, the hydrocarbon chain is non-polar and hydrophobic, repelling water. This duality enables fatty acids to form various structures in biological systems, such as:
- Triglycerides: Composed of three fatty acids esterified to a glycerol molecule, serving as energy storage in animals and plants.
- Phospholipids: Contain two fatty acids and a phosphate group attached to glycerol, forming the lipid bilayer of cell membranes.
- Micelles: Spherical aggregates of fatty acids in aqueous solutions, with hydrophobic tails inward and hydrophilic heads outward, facilitating the transport of lipids in the digestive system.
The length and degree of unsaturation of the hydrocarbon chain influence the physical properties of fatty acids, such as melting point and fluidity. Saturated fatty acids with longer chains tend to be solid at room temperature, while unsaturated fatty acids, especially those with cis double bonds, are typically liquid.
Understanding the structure and behavior of fatty acids is crucial in biochemistry and nutrition, as they play vital roles in energy storage, membrane structure, and signaling pathways within the body.