Shown Below Is The Structural Formula For Amino Acid Alanine. Complete The Lewis Structure By Drawing The Non-Bonding Electrons, And Answer The Next 2 Questions Based On This Information.

The Correct Answer and Explanation is :
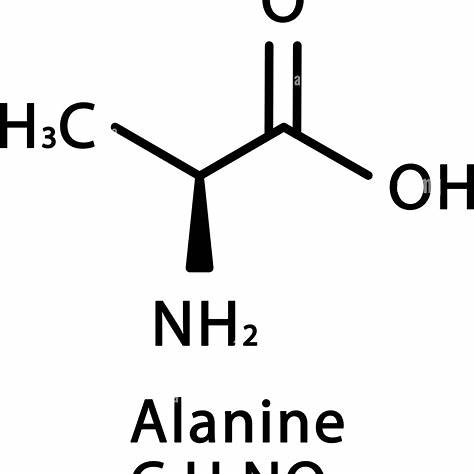
Alanine, an α-amino acid, is fundamental in protein synthesis and metabolism. Its structural formula is C₃H₇NO₂, comprising three carbon atoms, seven hydrogen atoms, one nitrogen atom, and two oxygen atoms.
Lewis Structure of Alanine:
To construct the Lewis structure of alanine, follow these steps:
- Identify Valence Electrons:
- Carbon (C): 4 valence electrons each × 3 atoms = 12 electrons
- Hydrogen (H): 1 valence electron each × 7 atoms = 7 electrons
- Nitrogen (N): 5 valence electrons
- Oxygen (O): 6 valence electrons each × 2 atoms = 12 electrons
- Total Valence Electrons: 12 (C) + 7 (H) + 5 (N) + 12 (O) = 36 electrons
- Arrange the Atoms:
- Place the nitrogen (N) atom at the center, bonded to a hydrogen (H) atom.
- Attach the central carbon (C) atom to the nitrogen, forming an amide group (–NH₂).
- Connect the central carbon to a carboxyl group (–COOH) and a methyl group (–CH₃).
- Distribute Electrons:
- Form single bonds between atoms, using two electrons per bond.
- Allocate remaining electrons to complete the octet for each atom, ensuring nitrogen has two lone pairs, and oxygen atoms have three lone pairs each.
Explanation:
Alanine’s structure consists of a central α-carbon bonded to:
- Amino Group (–NH₂): This group imparts basic properties to alanine.
- Carboxyl Group (–COOH): This group provides acidic characteristics.
- Methyl Side Chain (–CH₃): This nonpolar side chain classifies alanine as a non-essential, aliphatic amino acid.
Under physiological conditions, alanine exists as a zwitterion, where the amino group is protonated (–NH₃⁺), and the carboxyl group is deprotonated (–COO⁻), resulting in a net neutral molecule with internal charge separation.
This zwitterionic form enhances alanine’s solubility in water and influences its role in protein structures and functions.