For most substances, solubility increases as temperature increases. Can you find any exceptions on the graph? Find the exceptions to this and EXPLAIN how you know they are exceptions to this rule.
The Correct Answer and Explanation is :
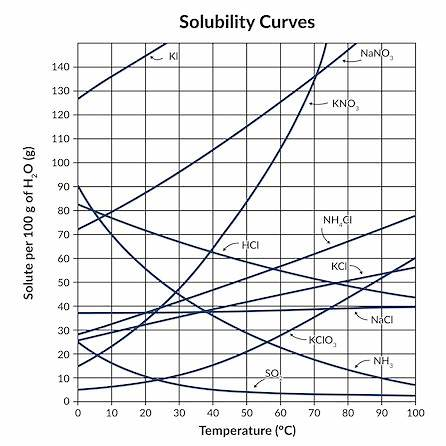
While it’s generally observed that the solubility of most solid substances increases with temperature, there are notable exceptions. One prominent example is sodium chloride (NaCl), commonly known as table salt. On a solubility vs. temperature graph, NaCl’s curve remains relatively flat, indicating that its solubility doesn’t significantly change with temperature. This behavior is attributed to the dissolution of NaCl being an endothermic process, meaning it absorbs heat. However, the enthalpy change (ΔH) is small, resulting in minimal variation in solubility across a range of temperatures.
Conversely, substances like potassium nitrate (KNO₃) exhibit a steeply rising solubility curve. For KNO₃, solubility increases significantly with temperature, a characteristic typical of many salts whose dissolution is endothermic with a substantial positive ΔH.
Additionally, certain salts, such as calcium sulfate (CaSO₄), display inverse solubility behavior. Their solubility decreases as temperature increases, a phenomenon known as retrograde solubility. This occurs because the dissolution process of these salts is exothermic (releases heat), and according to Le Chatelier’s principle, adding heat shifts the equilibrium toward the undissolved solid, reducing solubility.
These variations arise from the interplay between enthalpy and entropy changes during dissolution. For most salts, the process is endothermic, leading to increased solubility with rising temperature. However, for salts with exothermic dissolution processes, solubility decreases as temperature increases. Understanding these exceptions is crucial for applications requiring precise solubility control, such as in recrystallization processes and industrial formulations.