Which of the following statements is true concerning the electron configuration [Ne]3p2?
A.This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Heisenberg uncertainty principle.
B.This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Pauli exclusion principle.
C.This configuration is the ground-state electron configuration for a Mg atom.
D.This configuration cannot be the ground-state electron configuration for a Mg atom because it violates Hund’s rule.
E.This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Aufbau principle.
The Correct Answer and Explanation is :
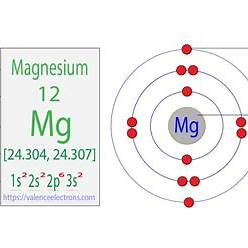
The correct answer is E. This configuration cannot be the ground-state electron configuration for a Mg atom because it violates the Aufbau principle.
Explanation:
The electron configuration [Ne]3p² suggests that after the neon core configuration ([Ne] represents 1s² 2s² 2p⁶), two electrons occupy the 3p subshell. However, magnesium (Mg) has an atomic number of 12, meaning it has 12 electrons in its neutral state. Following the Aufbau principle, which dictates that electrons fill orbitals starting from the lowest energy level, the electron configuration for Mg should be [Ne]3s². This configuration places two electrons in the 3s subshell, which is lower in energy than the 3p subshell. Therefore, the configuration [Ne]3p² is not possible for a ground-state Mg atom, as it violates the Aufbau principle by placing electrons in a higher-energy subshell (3p) before filling the lower-energy 3s subshell.
This configuration shows the arrangement of electrons in the 1s, 2s, 2p, and 3s orbitals, consistent with Mg’s position in the periodic table and the principles governing electron configurations.