Given this kinetic data from a reaction that obeys Michaelis-Menten kinetics:
k1 = 2×10^8 M/s, k-1 = 1×10^3 sec-1, k2 = 5×10^3 sec-1
Ks = 5.0×10^(-6) M, Km = 3.0×10^(-5)
What is Kcat and the catalytic efficiency (Kcat/Km) for this enzyme?
The correct answer and explanation is :
To determine the catalytic constant (Kcat) and the catalytic efficiency (Kcat/Km) for the enzyme, we can use the provided kinetic parameters.
Calculation of Kcat:
The catalytic constant, Kcat, also known as the turnover number, represents the maximum number of substrate molecules converted to product per enzyme molecule per second. It is equivalent to the rate constant k₂ in the Michaelis-Menten mechanism. Given that k₂ = 5 × 10³ s⁻¹, we have:
Kcat = k₂ = 5,000 s⁻¹
Calculation of Catalytic Efficiency (Kcat/Km):
The catalytic efficiency combines the enzyme’s turnover number (Kcat) and its affinity for the substrate (Km). It is calculated as:
Kcat/Km = (5,000 s⁻¹) / (3.0 × 10⁻⁵ M)
Performing the division:
Kcat/Km = 1.67 × 10⁸ M⁻¹s⁻¹
Explanation:
Enzymes are biological catalysts that accelerate chemical reactions by lowering the activation energy required for the reaction to proceed. The efficiency and specificity of an enzyme are often characterized by kinetic parameters such as Kcat, Km, and the ratio Kcat/Km.
- Kcat (Turnover Number): This parameter indicates the number of substrate molecules an enzyme can convert into product per second when the enzyme is fully saturated with substrate. In this case, a Kcat of 5,000 s⁻¹ means that each enzyme molecule can process 5,000 substrate molecules into product every second under optimal conditions. This high turnover number suggests a highly efficient catalytic process.
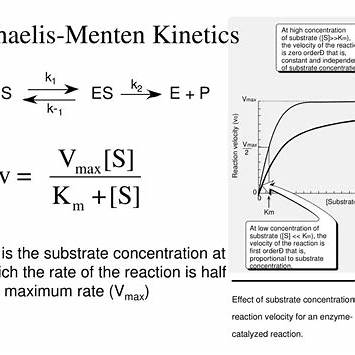
- Km (Michaelis Constant): Km reflects the substrate concentration at which the reaction rate is at half its maximum (Vmax). It provides insight into the affinity between the enzyme and its substrate; a lower Km indicates higher affinity, meaning the enzyme can achieve half-maximal catalysis at a lower substrate concentration. In this scenario, Km is given as 3.0 × 10⁻⁵ M, suggesting a moderate affinity between the enzyme and its substrate.
- Kcat/Km (Catalytic Efficiency): This ratio combines both the turnover number and the substrate affinity to provide a measure of the enzyme’s overall efficiency. It is particularly useful when comparing different enzymes or the same enzyme with different substrates. A higher Kcat/Km value indicates a more efficient enzyme. The calculated catalytic efficiency of 1.67 × 10⁸ M⁻¹s⁻¹ is quite high, approaching the diffusion limit (approximately 10⁸ to 10⁹ M⁻¹s⁻¹), which is the rate at which enzymes and substrates diffuse together in solution. Enzymes with catalytic efficiencies near this limit are considered to be “catalytically perfect,” as their rate of catalysis is limited only by the rate of substrate diffusion.
In summary, the enzyme exhibits a high turnover number and catalytic efficiency, indicating it is highly effective at converting substrate to product. Such enzymes are often involved in critical biochemical pathways where rapid and efficient catalysis is essential.