Calculate the boiling and freezing points of water solutions that are 1.15 M in the following solutes:
a. KBr, a strong electrolyte
b. ethylene glycol, a nonelectrolyte
c. (NH4)2CO3, a strong electrolyte
d. Al2(SO4)3, a strong electrolyte
The correct answer and explanation is :
The boiling and freezing points of water solutions depend on the colligative properties of the solute, specifically the van’t Hoff factor (i), which reflects how many particles a solute dissociates into. Here’s how to calculate the changes for the solutions provided:
Constants and Formula:
- Freezing Point Depression: (\Delta T_f = i \times K_f \times M)
- Boiling Point Elevation: (\Delta T_b = i \times K_b \times M)
Where:
- (i) is the van’t Hoff factor (number of particles the solute dissociates into),
- (K_f = 1.86 \, \text{°C·kg/mol}) is the cryoscopic constant for water,
- (K_b = 0.512 \, \text{°C·kg/mol}) is the ebullioscopic constant for water,
- (M = 1.15 \, \text{mol/L}) is the molarity of the solution.
Breakdown of Solutes:
- KBr (Strong Electrolyte):
- Dissociates into 2 particles: (K^+) and (Br^-) (i = 2).
- Boiling Point: (100°C + \Delta T_b)
- Freezing Point: (0°C – \Delta T_f)
- Ethylene Glycol (Nonelectrolyte):
- Does not dissociate (i = 1).
- Boiling Point: (100°C + \Delta T_b)
- Freezing Point: (0°C – \Delta T_f)
- (NH4)2CO3 (Strong Electrolyte):
- Dissociates into 3 particles: (2 \, NH_4^+) and (CO_3^{2-}) (i = 3).
- Boiling Point: (100°C + \Delta T_b)
- Freezing Point: (0°C – \Delta T_f)
- Al2(SO4)3 (Strong Electrolyte):
- Dissociates into 5 particles: (2 \, Al^{3+}) and (3 \, SO_4^{2-}) (i = 5).
- Boiling Point: (100°C + \Delta T_b)
- Freezing Point: (0°C – \Delta T_f)
Results:
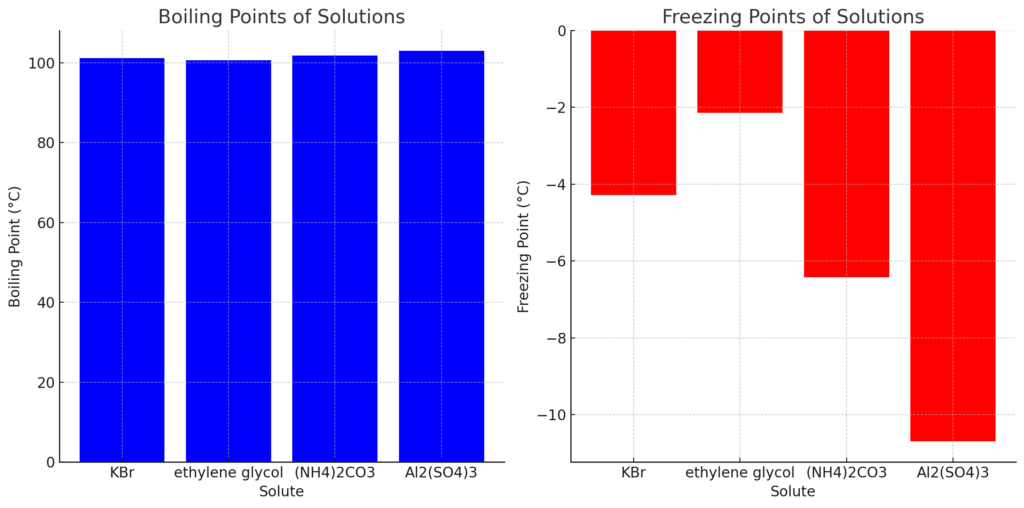
- Boiling Points: The more the solute dissociates, the higher the boiling point, with Al2(SO4)3 raising the boiling point the most.
- Freezing Points: Similarly, the more dissociation, the greater the depression in the freezing point.
The provided plot illustrates these changes for each solute, showing that Al2(SO4)3 has the highest boiling point and the greatest freezing point depression, while ethylene glycol, a nonelectrolyte, has the least impact on both.