Draw electron dot and cross diagrams for the following compounds. Only the outermost shell of electrons needs to be shown. Use a different dot or cross for each atom or ion.
(a) CO, carbon monoxide
(b) Al2Cl6, aluminum chloride dimer
The correct answer and explanation is :
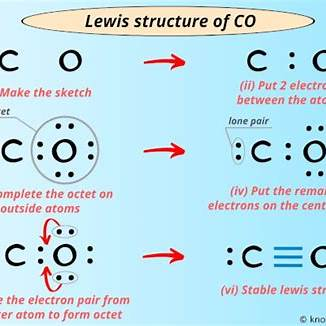
Electron dot and cross diagrams, also known as Lewis structures, visually represent the valence electrons of atoms within a molecule, illustrating how atoms bond to form compounds. In these diagrams, dots (•) and crosses (×) denote electrons, with each atom or ion’s electrons uniquely marked for clarity.
(a) Carbon Monoxide (CO):
Carbon monoxide consists of one carbon atom bonded to one oxygen atom. To depict its electron dot and cross diagram:
- Valence Electrons:
- Carbon (C): Group 14 element, has 4 valence electrons.
- Oxygen (O): Group 16 element, has 6 valence electrons.
- Total Valence Electrons: 4 (from C) + 6 (from O) = 10 valence electrons.
- Bond Formation:
- Carbon and oxygen atoms share electrons to achieve stable electron configurations.
- In CO, a triple bond forms between carbon and oxygen, consisting of three shared electron pairs.
- Additionally, both atoms possess one lone pair of electrons each.
- Diagram Representation:
- Carbon’s electrons are represented by crosses (×), and oxygen’s by dots (•)
- The triple bond is shown by three lines connecting the atoms, each line representing a shared electron pair.
- Lone pairs are placed adjacent to the respective atoms.
(b) Aluminum Chloride Dimer (Al₂Cl₆):
Aluminum chloride typically exists as a dimer (Al₂Cl₆) in the vapor phase, especially at lower temperatures, due to its electron-deficient nature. To illustrate its electron dot and cross diagram:
- Valence Electrons:
- Aluminum (Al): Group 13 element, has 3 valence electrons.
- Chlorine (Cl): Group 17 element, has 7 valence electrons.
- Total Valence Electrons: 2 × 3 (from Al) + 6 × 7 (from Cl) = 24 valence electrons.
- Bond Formation:
- Each aluminum atom forms coordinate (dative covalent) bonds with two chlorine atoms.
- A coordinate bond occurs when both electrons in the shared pair originate from the same atom.
- Chlorine atoms donate lone pairs to aluminum atoms, resulting in two Al-Cl-Al bridges.
- Diagram Representation:
- Aluminum’s electrons are marked with crosses (×), and chlorine’s with dots (•).
- Each Al-Cl-Al bridge is depicted with an arrow indicating the direction of electron donation.
- Lone pairs on chlorine atoms are shown adjacent to the chlorine symbols.
Understanding these diagrams aids in visualizing molecular structures and the distribution of electrons, which is fundamental to predicting chemical reactivity and properties.