How does an enzyme affect a chemical reaction? Select all that apply.
- Decreases the rate of the reaction
- Lowers the activation energy
- Increases the activation energy
- Increases the rate of the reaction
The correct answer and explanation is :
Enzymes are biological catalysts that significantly accelerate chemical reactions within the body by lowering the activation energy required for these reactions to proceed. This reduction in activation energy enables reactions to occur more rapidly and efficiently, which is essential for maintaining life processes.
Correct Answers:
- 2. Lowers the activation energy
- 4. Increases the rate of the reaction
Explanation:
- Lowering Activation Energy: Enzymes function by binding to substrates—the reactants in enzyme-catalyzed reactions—and forming an enzyme-substrate complex. This complex stabilizes the transition state, effectively lowering the activation energy needed for the reaction to occur. By providing an alternative reaction pathway with a lower energy threshold, enzymes enable reactions to proceed more swiftly.
- Increasing Reaction Rate: By lowering the activation energy, enzymes accelerate the rate at which reactions reach completion. This acceleration is crucial for sustaining the numerous biochemical processes that occur in living organisms, ensuring they proceed at rates sufficient to meet the body’s needs.
Incorrect Answers:
- Decreasing the Rate of the Reaction: Enzymes do not decrease reaction rates; rather, they enhance them by lowering the activation energy.
- Increasing the Activation Energy: Enzymes do not increase the activation energy; they lower it to facilitate faster reactions.
Visual Representation:
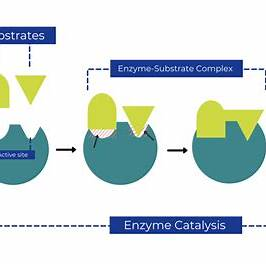
In summary, enzymes are essential for facilitating biochemical reactions by lowering activation energy barriers, which leads to an increased rate of reaction. This acceleration is vital for maintaining the efficiency and regulation of metabolic processes in living organisms.