What will happen to a red blood cell (RBC), which has an internal ion content of about 0.9%, if it is placed into a beaker of pure water?
A) The cell would shrink because the water in the beaker is hypotonic relative to the cytoplasm of the RBC.
B) The cell would swell because the water in the beaker is hypotonic relative to the cytoplasm of the RBC.
C) The cell would shrink because the water in the beaker is hypertonic relative to the cytoplasm of the RBC.
D) The cell will remain the same size because the solution outside the cell is isotonic.
The correct answer and explanation is :
The correct answer is:
B) The cell would swell because the water in the beaker is hypotonic relative to the cytoplasm of the RBC.
Explanation:
Red blood cells (RBCs) maintain an internal ion concentration of about 0.9% NaCl (sodium chloride). This concentration makes the cytoplasm of the RBC isotonic to physiological fluids such as blood plasma. When an RBC is placed in pure water, a stark difference in solute concentration is established between the inside of the cell and its surroundings.
In this scenario, pure water is hypotonic compared to the RBC’s cytoplasm because it has no solutes, while the RBC contains solutes (e.g., salts, proteins). Due to osmosis, water will naturally move from the area of lower solute concentration (outside the cell) to the area of higher solute concentration (inside the cell). This movement occurs through the RBC’s semi-permeable membrane.
As water enters the RBC, the cell swells due to the increased internal volume. If too much water enters, the RBC may burst (lyse) in a process known as hemolysis. This is why intravenous fluids given in medical settings must be carefully balanced in terms of tonicity to avoid causing damage to blood cells.
Key Concepts:
- Osmosis is the movement of water across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration.
- Hypotonic solutions cause cells to swell and potentially burst due to excessive water intake.
- Hypertonic solutions cause cells to shrink as water exits.
- Isotonic solutions maintain equilibrium, preventing net water movement.
Thus, placing an RBC in pure water will lead to swelling and potential bursting due to osmotic imbalance.
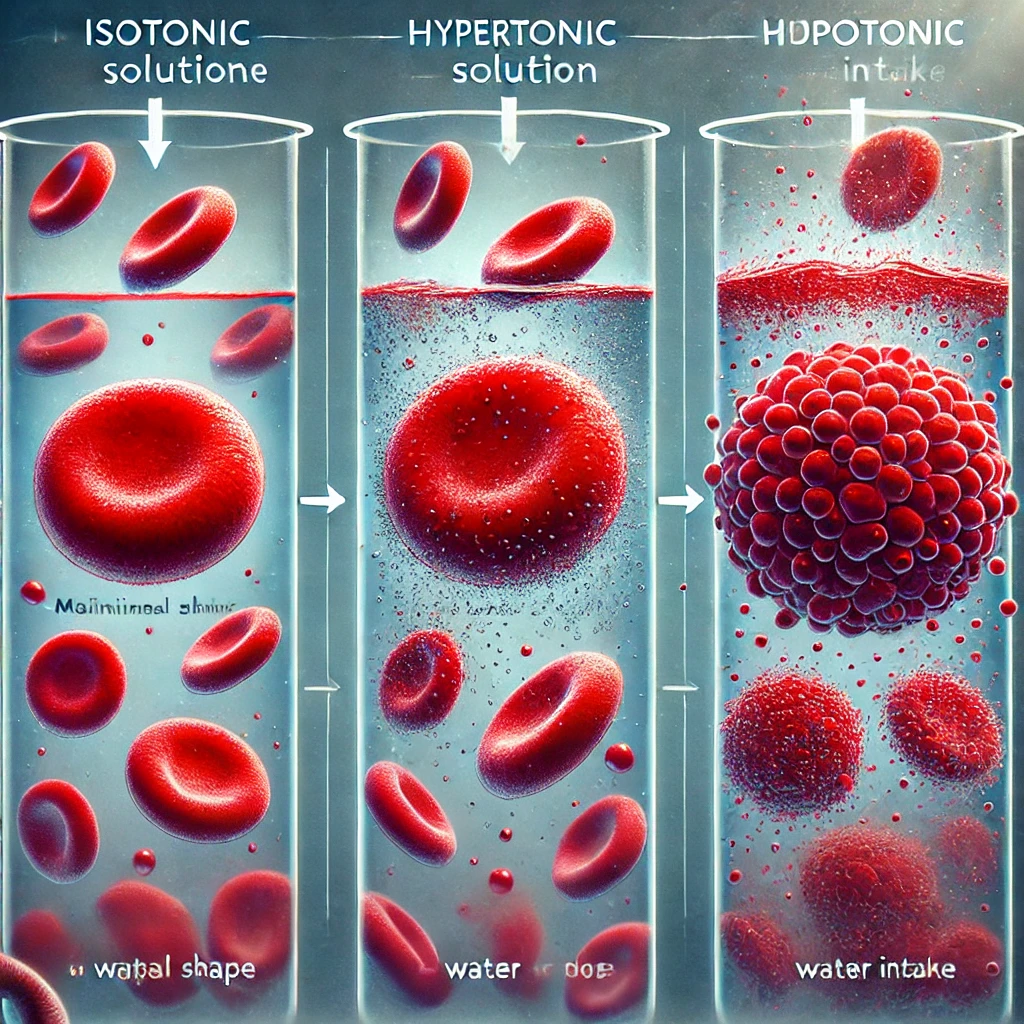
Here is an illustration showing the effect of osmosis on red blood cells. It depicts RBCs in isotonic, hypertonic, and hypotonic solutions, with arrows indicating water movement.