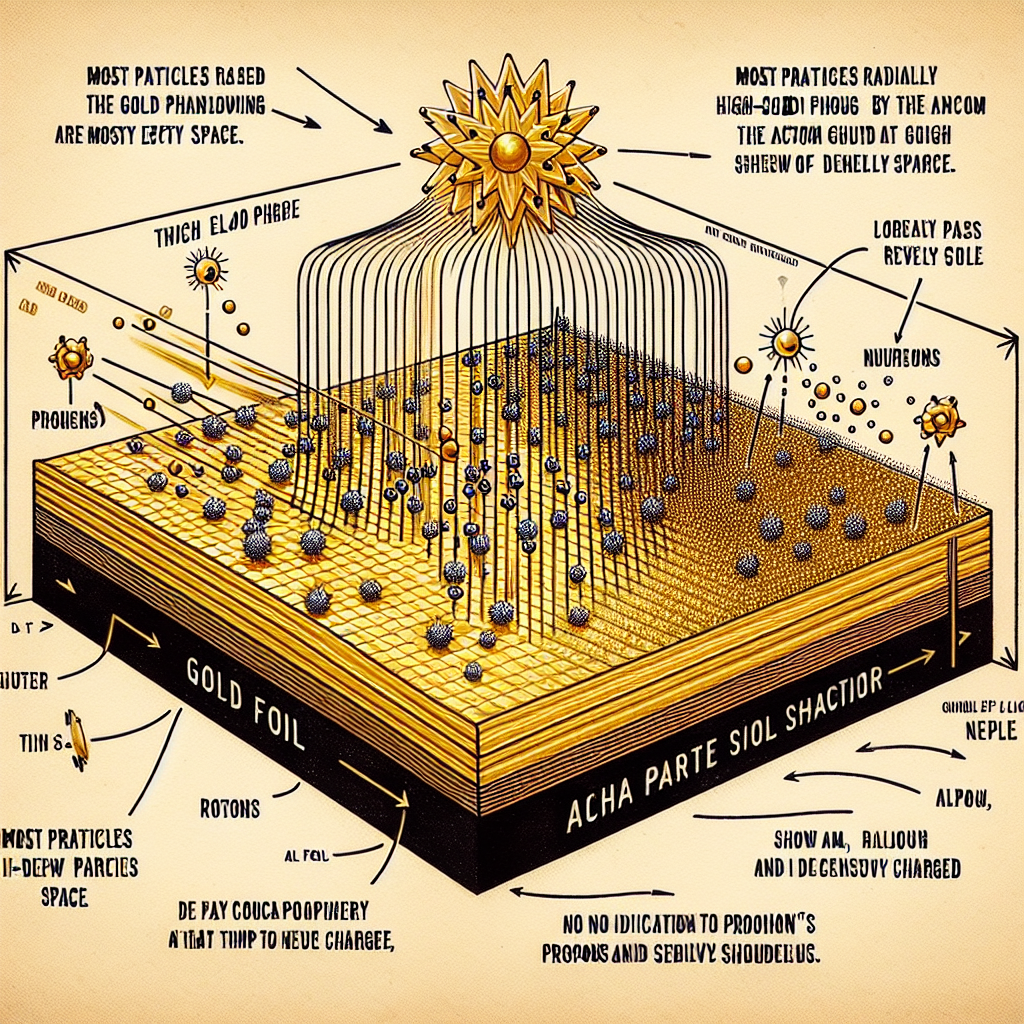
Which of the following was not a conclusion obtained from the gold foil-alpha particle scattering experiment?
A) An atom is mostly empty space.
B) An atom’s nucleus is positively charged.
C) An atom’s nucleus contains protons and neutrons.
D) Most of the mass of an atom is concentrated in its nucleus.
The correct answer and explanation is :
The correct answer is C) An atom’s nucleus contains protons and neutrons.
Explanation:
The gold foil-alpha particle scattering experiment was conducted by Ernest Rutherford in 1909. This experiment provided crucial insights into the structure of the atom and led to the development of the Rutherford model of the atom. The key findings from this experiment are:
- An atom is mostly empty space (Option A):
In the experiment, Rutherford directed a stream of alpha particles at a thin sheet of gold foil. Most of the alpha particles passed through the foil without deflection, suggesting that most of the atom is empty space. This result contradicted the earlier plum pudding model of the atom (proposed by J.J. Thomson), which suggested that the atom was a uniform “pudding” of positive charge with negative electrons embedded in it. - An atom’s nucleus is positively charged (Option B):
Some of the alpha particles were deflected at large angles, and a few even bounced back. This suggested that there was a small, dense region in the atom that repelled the positively charged alpha particles. Rutherford concluded that this region was the nucleus, and since the alpha particles were positively charged, the nucleus must be positively charged as well. - Most of the mass of an atom is concentrated in its nucleus (Option D):
The deflection of the alpha particles indicated the presence of a small, dense nucleus at the center of the atom. Since these particles were deflected by the nucleus, it was inferred that the mass of the atom was concentrated in this nucleus.
However, the experiment did not provide direct evidence for the presence of neutrons in the nucleus. Neutrons were discovered later by James Chadwick in 1932. Therefore, the conclusion that “an atom’s nucleus contains protons and neutrons” (Option C) was not a direct result of the gold foil experiment, and that’s why it is the correct answer.
In summary, while Rutherford’s experiment led to key conclusions about the structure of the atom, the identification of neutrons came after the experiment and was not part of the findings.