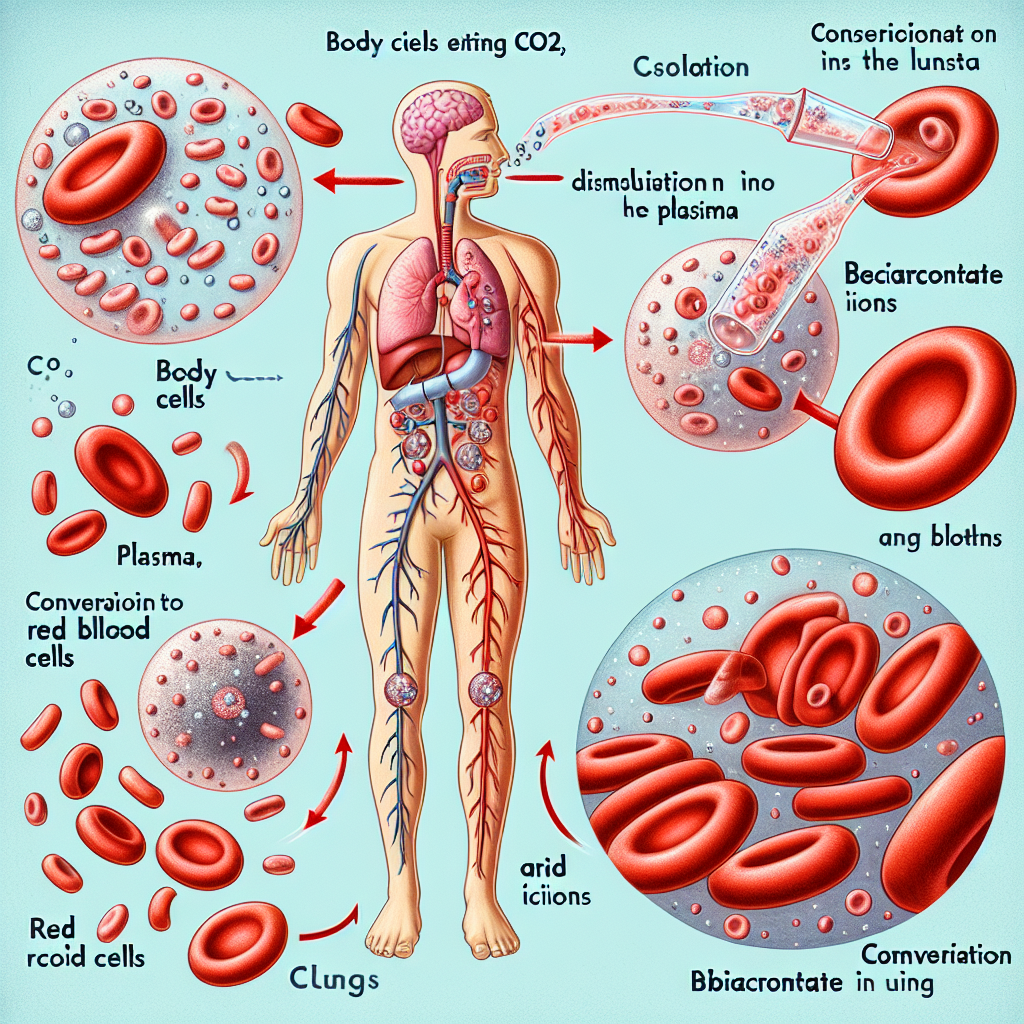
How is CO2 transported in blood?
The correct answer and explanation is :
How is CO₂ Transported in Blood?
Carbon dioxide (CO₂) is transported in the blood through three primary mechanisms:
- Dissolved in Plasma (7-10%) – A small percentage of CO₂ dissolves directly in the plasma and is carried in its free form.
- Bound to Hemoglobin (20-30%) – CO₂ binds to hemoglobin (Hb) in red blood cells, forming carbaminohemoglobin (HbCO₂). This process occurs at the amino groups of hemoglobin, not at the oxygen-binding sites.
- As Bicarbonate Ions (HCO₃⁻) (60-70%) – The majority of CO₂ is transported as bicarbonate. Inside red blood cells, CO₂ reacts with water (H₂O) under the action of the enzyme carbonic anhydrase, forming carbonic acid (H₂CO₃). This acid quickly dissociates into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺). The bicarbonate diffuses into the plasma, while chloride ions (Cl⁻) move into the RBCs to maintain electrical balance, a process called the chloride shift.
Explanation (300 words)
Carbon dioxide (CO₂) is a metabolic waste product produced by cells during aerobic respiration. Once formed, it must be efficiently transported to the lungs for exhalation. The three mechanisms of CO₂ transport ensure this happens efficiently.
A small fraction (7-10%) of CO₂ dissolves directly into the plasma and contributes to the blood’s partial pressure of CO₂. However, because CO₂ is more soluble than oxygen in water, this fraction plays a minor role in overall transport.
About 20-30% of CO₂ binds to hemoglobin in red blood cells. This occurs at the protein portion of hemoglobin, forming carbaminohemoglobin (HbCO₂). Importantly, this binding is reversible, allowing CO₂ to be released in the lungs.
The majority of CO₂ (60-70%) is carried as bicarbonate (HCO₃⁻). Within RBCs, the enzyme carbonic anhydrase facilitates the reaction:
[
CO₂ + H₂O \leftrightarrow H₂CO₃ \leftrightarrow HCO₃⁻ + H⁺
]
Bicarbonate diffuses out into the plasma, and to maintain electrical neutrality, chloride ions (Cl⁻) enter the RBCs in exchange. This is known as the chloride shift.
In the lungs, the process reverses: bicarbonate re-enters RBCs, reforms CO₂, and is exhaled. This system efficiently maintains acid-base balance and facilitates CO₂ removal.
Image of CO₂ Transport in Blood