How is the bulk of carbon dioxide carried in blood?
The correct answer and explanation is :
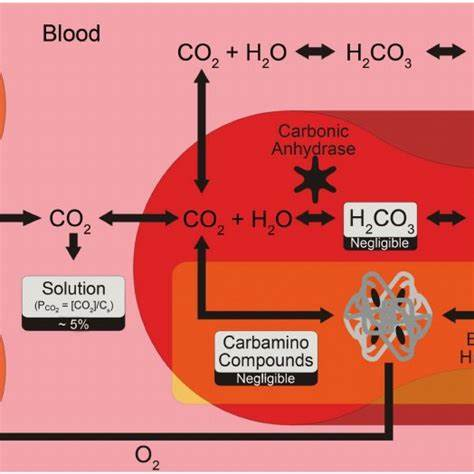
The majority of carbon dioxide (CO₂) in the blood is transported as bicarbonate ions (HCO₃⁻), accounting for approximately 60-70% of CO₂ transport.
When CO₂ diffuses into red blood cells (RBCs), it combines with water (H₂O) to form carbonic acid (H₂CO₃). This reaction is catalyzed by the enzyme carbonic anhydrase. Carbonic acid then quickly dissociates into hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻):
CO₂ + H₂O → H₂CO₃ → H⁺ + HCO₃⁻
Most of the bicarbonate ions diffuse out of the RBCs into the plasma. To maintain electrical neutrality, chloride ions (Cl⁻) move from the plasma into the RBCs, a process known as the chloride shift or Hamburger shift.
The hydrogen ions produced bind to hemoglobin, forming deoxyhemoglobin, which helps buffer the blood and facilitates oxygen release to tissues (the Bohr effect). When blood reaches the lungs, oxygen binds to hemoglobin, promoting the release of H⁺ ions. These hydrogen ions react with bicarbonate ions to form carbonic acid, which then dissociates into CO₂ and water. The CO₂ is then exhaled.
In summary, the conversion of CO₂ to bicarbonate ions within RBCs, facilitated by carbonic anhydrase, is the primary mechanism for CO₂ transport in the blood. This system efficiently manages CO₂ levels and maintains acid-base balance in the body.