Which of the following gases has the greatest Henry’s law constant in water at 25°C: CH4, Ne, HCl, H2?
The correct answer and explanation is :
Among the gases listed—methane (CH₄), neon (Ne), hydrogen chloride (HCl), and hydrogen (H₂)—hydrogen chloride (HCl) has the greatest Henry’s law constant in water at 25°C.
Henry’s law describes the solubility of a gas in a liquid at a constant temperature, stating that the amount of dissolved gas is directly proportional to its partial pressure above the liquid. The proportionality constant, known as Henry’s law constant (k_H), varies for different gases and is influenced by factors such as temperature and the nature of the gas-liquid interaction.
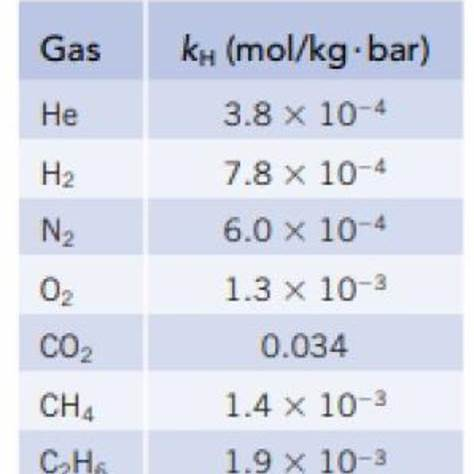
For the gases in question, the Henry’s law constants in water at 25°C are as follows:
- Hydrogen (H₂): 0.00078 mol/(L·atm)
- Neon (Ne): 0.00045 mol/(L·atm)
- Methane (CH₄): Approximately 0.0014 mol/(L·atm)
- Hydrogen chloride (HCl): Significantly higher than the other gases listed
While specific values for HCl’s Henry’s law constant are not provided in the available sources, it is well-known that HCl is highly soluble in water, reacting to form hydrochloric acid. This reaction greatly increases its solubility compared to non-reactive gases like H₂, Ne, and CH₄.