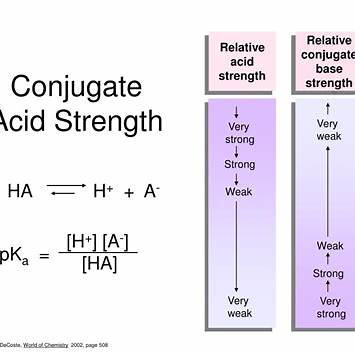
The stronger the acid, the __ the conjugate base.
A) stronger
B) weaker
The correct answer and explanation is :
The correct answer is:
B) weaker
Explanation:
Acids and bases exist in conjugate pairs, meaning that every acid has a corresponding conjugate base. The strength of an acid is determined by its ability to donate protons (H⁺ ions). A strong acid dissociates completely in water, meaning it releases all of its protons, leaving behind a very weak conjugate base.
Why Does a Strong Acid Have a Weak Conjugate Base?
- Dissociation of Strong Acids:
Strong acids such as hydrochloric acid (HCl) or sulfuric acid (H₂SO₄) ionize completely in solution:
[
HCl \rightarrow H^+ + Cl^-
]
Since HCl donates protons so easily, its conjugate base (Cl⁻) has almost no tendency to reaccept protons, making it a very weak base. - Stability of the Conjugate Base:
The conjugate base of a strong acid is usually very stable. Stability reduces the tendency to accept protons, making the base weaker. For example, sulfate ions (SO₄²⁻) from H₂SO₄ are stable and do not easily react to form H₂SO₄ again. - Equilibrium Considerations:
The acid-base strength relationship is governed by equilibrium principles. The stronger an acid, the more the reaction favors the formation of H⁺ and the conjugate base, leaving behind a base that is less reactive. Conversely, a weak acid like acetic acid (CH₃COOH) has a stronger conjugate base (CH₃COO⁻), meaning it can reaccept protons more readily.
Thus, the stronger the acid, the weaker its conjugate base because the base is less likely to react to form the original acid again.