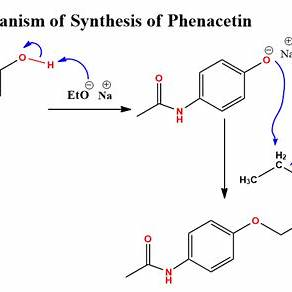
The Williamson ether synthesis proceeds via an
A) SN1
B) SN2 mechanism
C) E1
D) E2
E) none of the above
The correct answer and explanation is :
The correct answer is:
B) SN2 mechanism
Explanation:
The Williamson ether synthesis is a widely used method for preparing ethers. It involves the reaction of an alkoxide ion (RO⁻) with a primary alkyl halide (R’X) or tosylate (R’OTs) via an S(_N2) (bimolecular nucleophilic substitution) mechanism.
Mechanism of the Williamson Ether Synthesis (SN2)
- Generation of the Nucleophile (Alkoxide Ion)
- The reaction starts with the formation of an alkoxide ion (RO⁻), typically by deprotonating an alcohol (ROH) using a strong base such as sodium hydride (NaH) or sodium metal (Na).
- ( ROH + NaH \rightarrow RO^- + H_2 )
- Nucleophilic Attack on Alkyl Halide
- The alkoxide ion (RO⁻), a strong nucleophile, attacks the electrophilic carbon of an alkyl halide (R’X) in a backside attack, displacing the leaving group (X⁻).
- This occurs in a single step, characteristic of an S(_N2) reaction.
- Formation of the Ether Product
- The result is the formation of an ether (ROR’) and the departure of the halide ion (X⁻).
- ( RO^- + R’-X \rightarrow R-O-R’ + X^- )
Why is the Williamson Ether Synthesis SN2?
- Backside Attack: The reaction follows a one-step bimolecular mechanism where the nucleophile attacks from the opposite side, leading to inversion of configuration if the carbon is chiral.
- Best with Primary Alkyl Halides: SN2 reactions occur more efficiently with primary alkyl halides because steric hindrance is minimal. Secondary alkyl halides react more slowly, and tertiary alkyl halides typically undergo elimination instead.
- Strong Nucleophile: The alkoxide ion is a strong nucleophile, favoring the SN2 pathway.
Limitations of the Williamson Ether Synthesis
- Not effective for tertiary alkyl halides because they undergo E2 elimination instead.
- Works best for primary and some secondary alkyl halides.