What is the hybridization of XeO2 and XeO3?
The correct answer and explanation is :
Hybridization of XeO₂ and XeO₃
- XeO₂ (Xenon Dioxide): The hybridization of Xe in XeO₂ is sp³.
- XeO₃ (Xenon Trioxide): The hybridization of Xe in XeO₃ is sp³.
Explanation
Xenon oxides are interesting compounds because xenon, a noble gas, forms bonds with oxygen despite having a full valence shell. The hybridization in these compounds depends on the number of bonding and lone pairs around the xenon atom.
1. Hybridization of Xe in XeO₂
- Xenon (Xe) has 8 valence electrons.
- Oxygen atoms form double bonds with xenon. Each double bond uses 2 electrons per bond (total of 4 electrons).
- Remaining 4 electrons (or 2 lone pairs) stay on Xe.
- The steric number (bonding regions + lone pairs) = 4 (2 sigma bonds + 2 lone pairs).
- A steric number of 4 corresponds to sp³ hybridization.
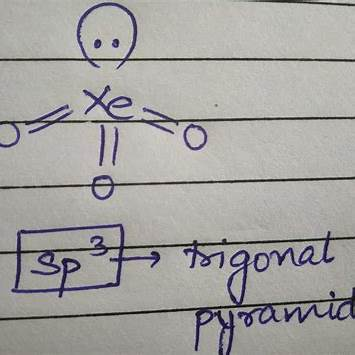
2. Hybridization of Xe in XeO₃
- Xenon has 8 valence electrons.
- Three oxygens form double bonds with xenon, utilizing 6 electrons.
- The remaining 2 electrons form one lone pair on Xe.
- The steric number = 4 (3 sigma bonds + 1 lone pair).
- A steric number of 4 means sp³ hybridization.
Molecular Geometry
- XeO₂ has a bent (V-shaped) structure due to lone pair repulsions.
- XeO₃ has a trigonal pyramidal shape because of its lone pair repulsion.