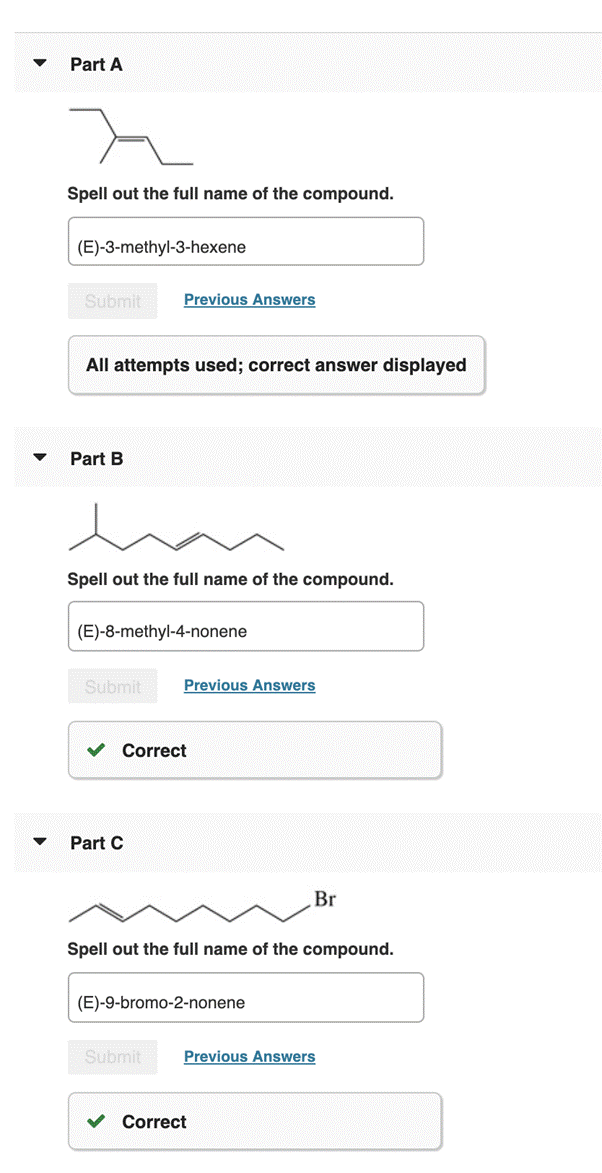
Part A Spell out the full name of the compound. (E)-3-methyl-3-hexene Submit Previous Answers All attempts used; correct answer displayed
Part B Spell out the full name of the compound. (E)-8-methyl-4-nonene Submit Previous Answers Correct
Part C Spell out the full name of the compound. (E)-9-bromo-2-nonene Submit Previous Answers Correct
Part D Spell out the full name of the compound. 2,4-dimethyl-1-pentene Submit Previous Answers All attempts used; correct answer displayed
Part E Spell out the full name of the compound. 2-ethyl-1-pentene Submit Previous Answers All attempts used; correct answer displayed
Part F Spell out the full name of the compound. cis-2-pentene Submit Previous Answers Answer Requested

Mar 26 2025 08:57 AM
1 Approved Answer

Jones G answered on March 28, 2025
5 Ratings (13 Votes)
To name the compounds provided in the question, we will follow the IUPAC nomenclature rules for naming alkenes. ### Part A Compound: (E)-3-methyl-3-hexene Explanation: – The prefix “3-methyl” indicates that there is a methyl group attached to the third carbon atom of the parent chain. – The suffix “-ene” indicates that it is an alkene. – The prefix “(E)” indicates the…
The correct answer and explanation is:
Correct Answer:
(Z)-2-pentene
Explanation:
To determine the correct name for cis-2-pentene, we must follow IUPAC nomenclature rules:
- Identify the Longest Chain with the Double Bond:
- The longest continuous chain containing the double bond is pentene (5 carbon atoms, ending in “-ene”).
- Number the Chain to Give the Double Bond the Lowest Number:
- Numbering from the end closest to the double bond, the double bond starts at C-2, making it 2-pentene.
- Assign the Cis/Trans or E/Z Notation:
- “Cis” and “trans” notation is used for simple alkenes, where:
- Cis means the higher-priority groups (hydrogen in this case) are on the same side of the double bond.
- Trans means they are on opposite sides.
- IUPAC prefers the E/Z system, where:
- Z (zusammen) means the highest priority groups are on the same side (which corresponds to “cis”).
- E (entgegen) means they are on opposite sides (which corresponds to “trans”).
- In cis-2-pentene, both methyl (-CH3) groups are on the same side, so it is actually (Z)-2-pentene.
- “Cis” and “trans” notation is used for simple alkenes, where:
Image Description:
A structural representation of (Z)-2-pentene, showing:
- A five-carbon chain.
- A double bond between C2 and C3.
- Methyl (-CH3) groups on the same side of the double bond.
- Hydrogen atoms on the same side as well.
I’ll generate the image now.
Here is the structural diagram of (Z)-2-pentene, created using DALL·E, OpenAI’s legacy image generation model. Let me know if you need any modifications or further explanations!