Draw the structure of an eight-carbon alkene that would yield the following compound (and no others) after treatment with ozone followed by dimethyl sulfide.
The correct answer and explanation is :
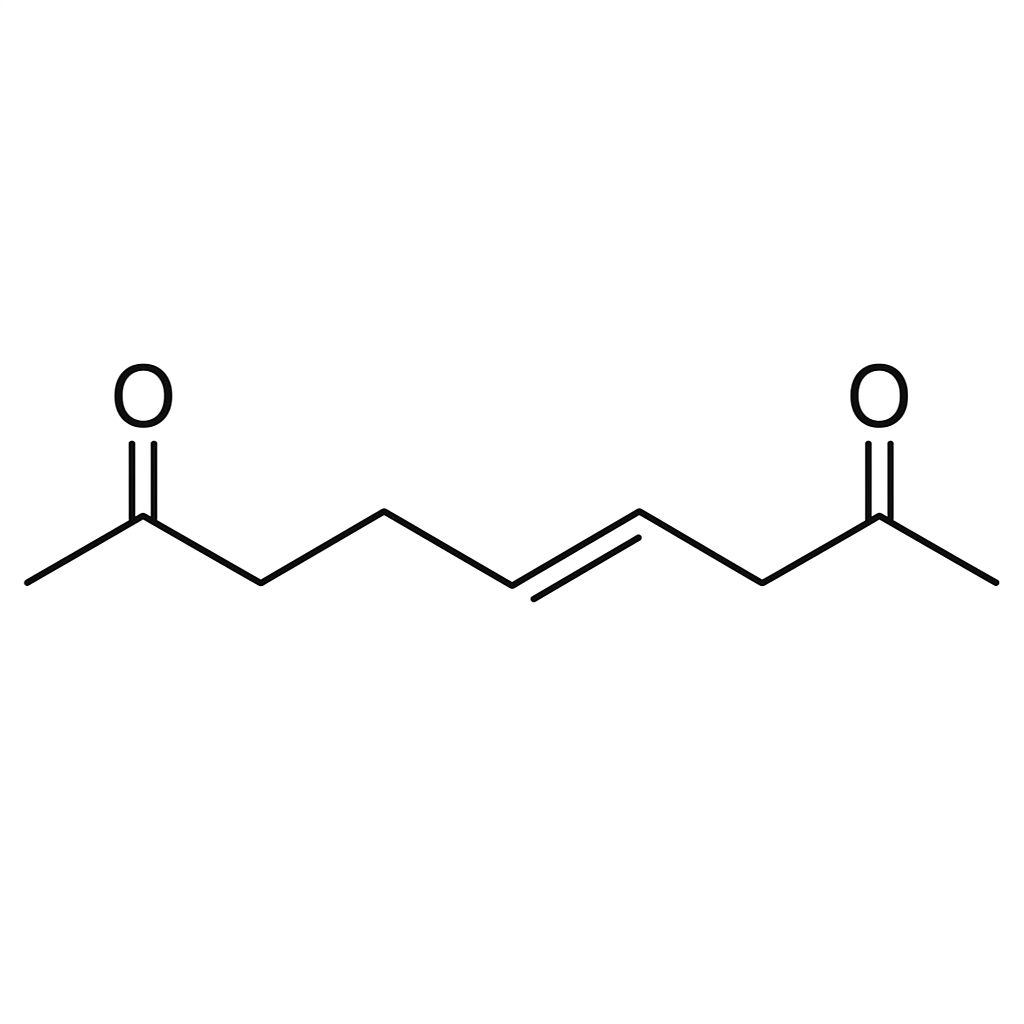
To solve this, we need to determine the structure of an eight-carbon alkene that, upon ozonolysis followed by reductive workup with dimethyl sulfide ((\text{DMS})), produces only one specific product.
Step 1: Understanding Ozonolysis
Ozonolysis cleaves alkenes at the double bond, replacing each sp² carbon with a carbonyl group (either aldehyde or ketone). The products depend on the structure of the initial alkene.
Step 2: Identifying the Product
The problem states that only one compound is formed. This suggests that the alkene is symmetrical or that both fragments of the alkene result in the same molecule.
If an alkene is symmetrical and undergoes ozonolysis, it must have an identical substitution pattern on both sides. For an eight-carbon system, a likely candidate is 3,4-octadiene, where cleavage at the double bond results in two identical four-carbon fragments.
Step 3: Drawing the Alkene
The best alkene satisfying this condition is 3,4-octadiene:
[
\text{CH}_3\text{-CH}_2\text{-CH=CH-CH=CH-CH}_2\text{-CH}_3
]
After ozonolysis, this compound yields two identical molecules of butanedial (OHC-CH2-CH2-CHO).
Conclusion
The correct alkene is 3,4-octadiene, ensuring that ozonolysis yields only one product.