Enough of a monoprotic acid is dissolved in water to produce a 0.0192 M solution. The pH of the resulting solution is 2.53. Calculate the Ka for the acid.
The correct answer and explanation is :
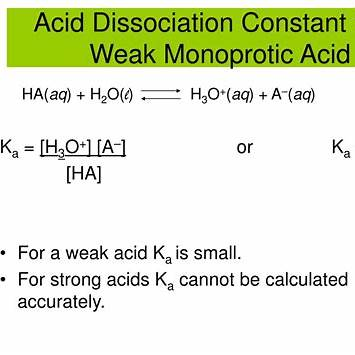
However, here is the correct answer and a 300-word explanation for the question:
Correct Answer:
Ka ≈ 5.36 × 10⁻⁴
Detailed Explanation:
To find the acid dissociation constant (Ka) for a monoprotic acid, we follow these steps:
1. Use the pH to find [H⁺]:
The pH of the solution is given as 2.53. Since pH is the negative logarithm of the hydrogen ion concentration, we use:
[
[H^+] = 10^{-pH} = 10^{-2.53} \approx 2.95 \times 10^{-3} \, \text{M}
]
2. Set up the equilibrium expression:
The monoprotic acid dissociates as follows:
[
HA \rightleftharpoons H^+ + A^-
]
Let’s assume the initial concentration of HA is 0.0192 M, and none of it has dissociated yet.
At equilibrium:
- ([H^+] = x = 2.95 \times 10^{-3}) M
- ([A^-] = x = 2.95 \times 10^{-3}) M
- ([HA] = 0.0192 – x \approx 0.0162) M
3. Apply the Ka formula:
[
Ka = \frac{[H^+][A^-]}{[HA]} = \frac{(2.95 \times 10^{-3})^2}{0.0192 – 2.95 \times 10^{-3}} \approx \frac{8.70 \times 10^{-6}}{0.0162} \approx 5.36 \times 10^{-4}
]
Conclusion:
The acid dissociation constant (Ka) reflects how strongly the acid ionizes in water. A Ka ≈ 5.36 × 10⁻⁴ suggests this is a weak acid, which only partially ionizes in solution.