The reason specific gravity is often cited rather than density is that:
Specific gravity has units that are consistent with the SI (metric) system.
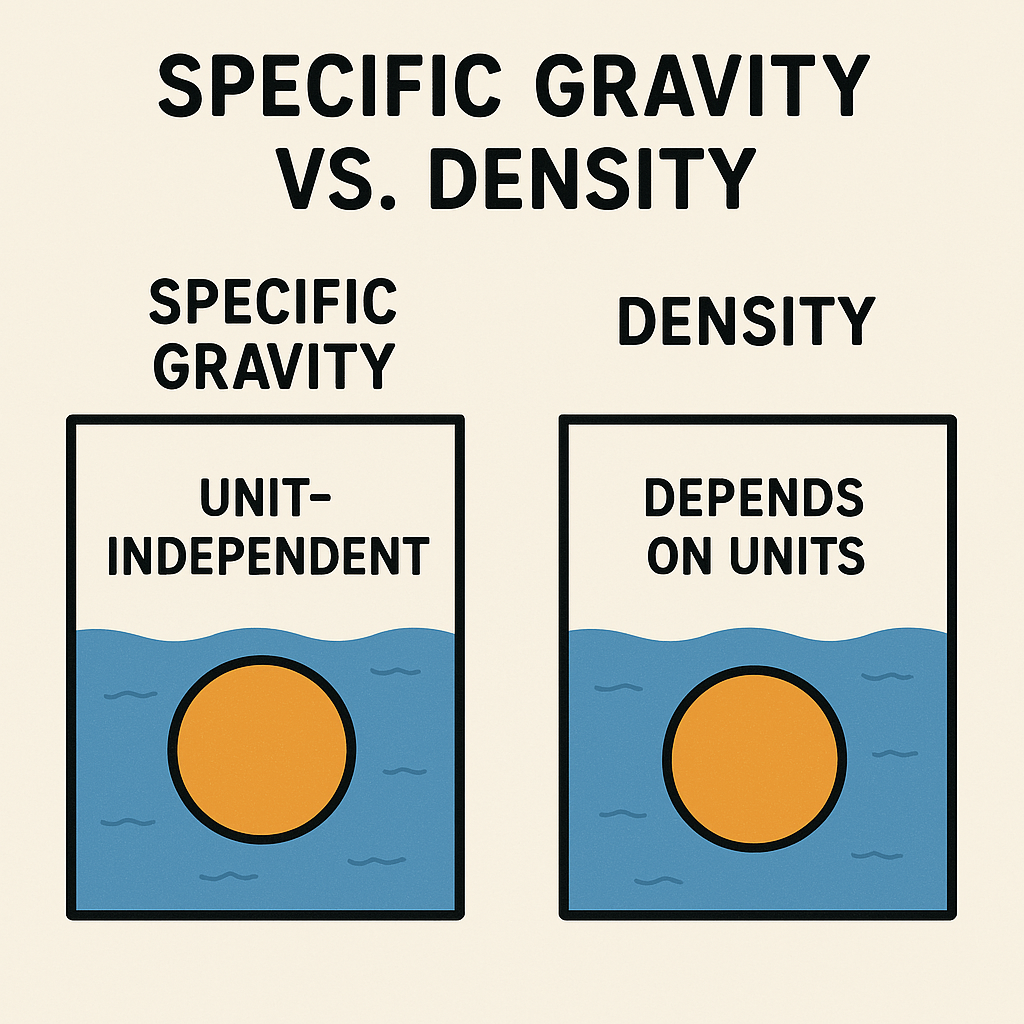
The numerical value of density depends on the mass and volume units chosen, whereas specific gravity is unit-independent.
The specific gravity of bodily fluids is always close to 1.
If the specific gravity of a substance is greater than 1, you know it will float on water.
The correct answer and explanation is :
Correct Answer:
The numerical value of density depends on the mass and volume units chosen, whereas specific gravity is unit-independent.
Explanation:
Specific gravity (SG) is a dimensionless quantity that expresses the ratio of the density of a substance to the density of a reference substance, typically water at 4°C for liquids and solids. Since it is a ratio of two densities, the units cancel out, making specific gravity unitless. This characteristic is one of the primary reasons why specific gravity is often used instead of density, especially in scientific, medical, and engineering contexts.
Density, on the other hand, is defined as mass per unit volume and is usually expressed in units like kilograms per cubic meter (kg/m³) in SI or grams per cubic centimeter (g/cm³) in the metric system. Its numerical value can vary significantly depending on the units used. For instance, the density of water is approximately 1000 kg/m³ or 1 g/cm³ — both correct but numerically different due to unit changes. This can lead to confusion or conversion errors if not carefully handled.
In contrast, specific gravity offers a straightforward comparison. For example, if a liquid has a specific gravity of 1.2, it is 1.2 times denser than water, regardless of the units used to measure mass and volume. This simplicity makes SG especially useful in industries like brewing, medicine (e.g., urine analysis), and geology, where relative densities are more important than exact values.
The other options are incorrect or misleading:
- Specific gravity has no units, so it isn’t tied to the SI system.
- Bodily fluids often have SG close to 1, but this is a contextual observation, not a reason SG is cited more often.
- If SG is greater than 1, the substance sinks in water, not floats — the reverse of what was stated.
Therefore, the unit-independence of specific gravity is the key reason it is frequently used.