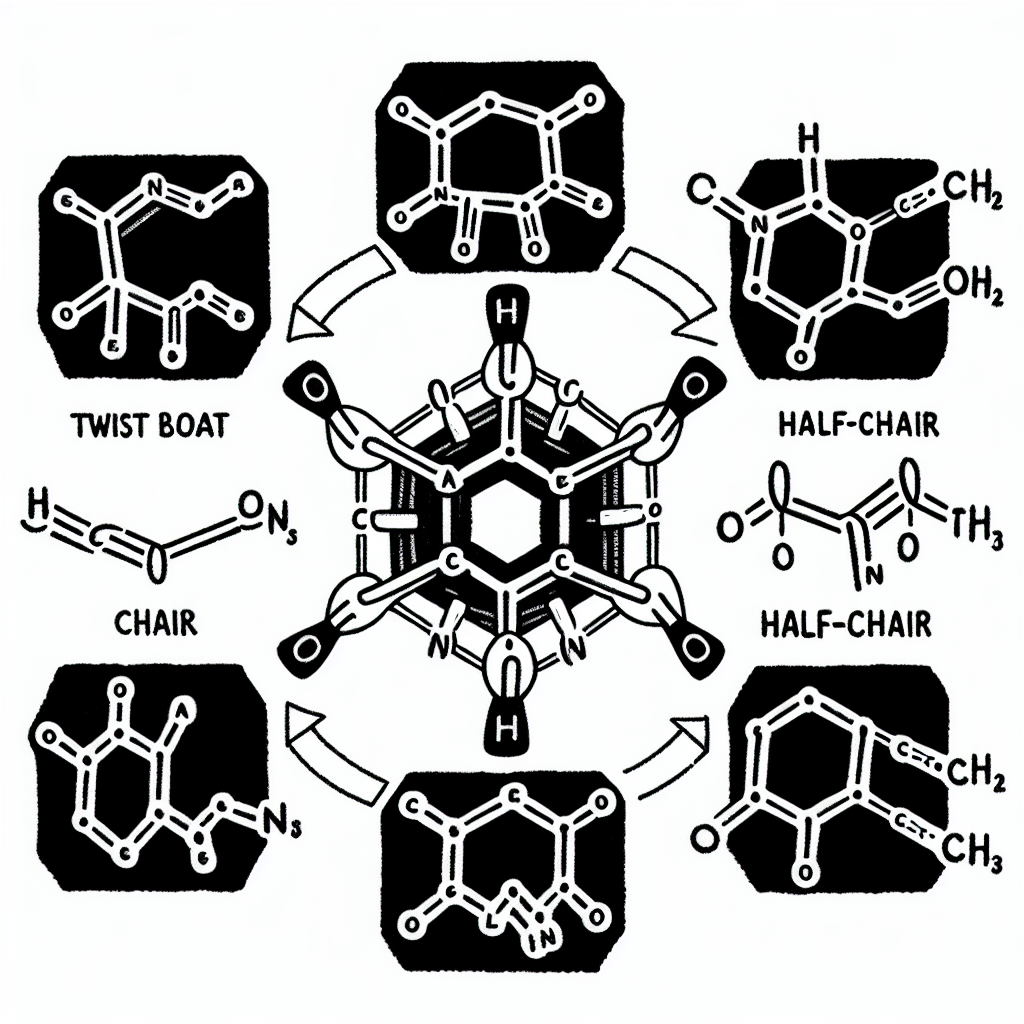
The least stable conformation of cyclohexane is the
A) boat
B) twist boat.
C) Chair
D) half-chair
E) twist chair.
The correct answer and explanation is :
The correct answer is: D) half-chair
Explanation (300 words):
Cyclohexane is a six-membered ring that adopts various conformations to minimize angle strain and torsional strain. Among these, the most well-known conformations are chair, boat, twist-boat, and half-chair. Each conformation has a different level of stability, primarily influenced by how well the molecule avoids eclipsing interactions and how close it stays to ideal bond angles (109.5° for sp³ carbons).
- Chair conformation is the most stable due to complete staggered interactions and minimal angle strain. All carbon-carbon bonds are staggered, which minimizes torsional strain. This is the default, low-energy conformation for cyclohexane.
- Boat conformation is less stable than chair because it introduces eclipsing interactions and steric hindrance between the two flagpole hydrogen atoms (those pointing toward each other at the top of the boat). However, there is no angle strain in the boat form.
- Twist-boat conformation is slightly more stable than the boat because it relieves some torsional strain by twisting the boat shape, reducing eclipsing interactions.
- Half-chair conformation is the least stable of all because it represents a high-energy transition state between chair and twist-boat forms. In this conformation, one carbon is lifted out of the plane, causing severe torsional and angle strain. It is not a stable, isolable form but rather an intermediate seen during ring flips and conformational changes.
- Twist-chair is not a standard term in conformational analysis of cyclohexane, and likely a distractor in the list.
Therefore, half-chair is the highest-energy, least stable conformation due to its combination of angle and torsional strain, making option D the correct choice.