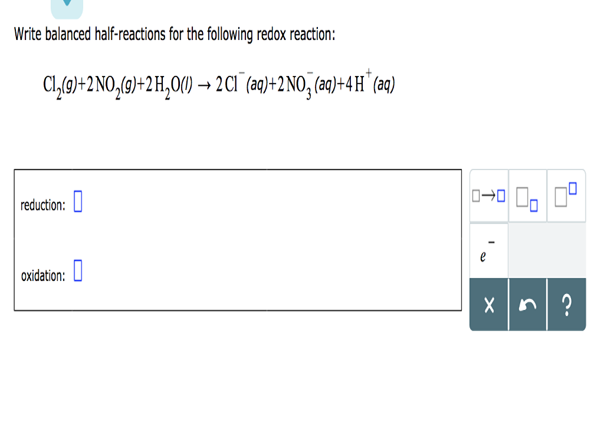
Write balanced half-reactions for the following redox reaction:
The Correct Answer and Explanation is:
Balanced Half-Reactions:
Reduction half-reaction:
Cl2(g)+2e−→2Cl−(aq)\text{Cl}_2(g) + 2e^- \rightarrow 2\text{Cl}^-(aq)
Oxidation half-reaction:
2NO2(g)+2H2O(l)→2NO3−(aq)+4H+(aq)+2e−2\text{NO}_2(g) + 2\text{H}_2O(l) \rightarrow 2\text{NO}_3^-(aq) + 4\text{H}^+(aq) + 2e^-
Explanation
Redox (reduction-oxidation) reactions involve the transfer of electrons between chemical species. In any redox reaction, one species loses electrons (oxidation), while another gains electrons (reduction). To analyze the given redox reaction: Cl2(g)+2NO2(g)+2H2O(l)→2Cl−(aq)+2NO3−(aq)+4H+(aq)\text{Cl}_2(g) + 2\text{NO}_2(g) + 2\text{H}_2O(l) \rightarrow 2\text{Cl}^-(aq) + 2\text{NO}_3^-(aq) + 4\text{H}^+(aq)
we must identify which species are oxidized and which are reduced.
Step 1: Assign oxidation states.
- In Cl2\text{Cl}_2, chlorine is in its elemental form: oxidation state = 0.
- In Cl−\text{Cl}^-, oxidation state = -1.
- Chlorine goes from 0 to -1: gain of electrons → reduction.
Hence, the reduction half-reaction is: Cl2(g)+2e−→2Cl−(aq)\text{Cl}_2(g) + 2e^- \rightarrow 2\text{Cl}^-(aq)
- In NO2\text{NO}_2, nitrogen has an oxidation state of +4.
- In NO3−\text{NO}_3^-, nitrogen has an oxidation state of +5.
- Nitrogen goes from +4 to +5: loss of electrons → oxidation.
So the oxidation half-reaction is: 2NO2(g)+2H2O(l)→2NO3−(aq)+4H+(aq)+2e−2\text{NO}_2(g) + 2\text{H}_2O(l) \rightarrow 2\text{NO}_3^-(aq) + 4\text{H}^+(aq) + 2e^-
Step 2: Balance mass and charge.
Each half-reaction is balanced for atoms and charges. The electrons cancel when the two reactions are added together: Cl2+2e−→2Cl−2NO2+2H2O→2NO3−+4H++2e−\text{Cl}_2 + 2e^- \rightarrow 2\text{Cl}^- \\ 2\text{NO}_2 + 2\text{H}_2O \rightarrow 2\text{NO}_3^- + 4\text{H}^+ + 2e^-
Combining both yields the balanced redox equation.
This systematic breakdown helps in understanding the flow of electrons and balancing redox equations correctly.
